
Exhibit 99.1

www.matinasbiopharma.com OTCQB: MTNB TRANSFORMING THE WAY POTENT MEDICINES FOR INFECTIOUS DISEASES ARE DESIGNED Corporate Presentation September 2015

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - looking statements. Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could,” “believes,” “estimates” and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or at all, including th e additional capital which will be necessary to complete the clinical trials of our product candidates; our ability to successf ull y complete research and further development and commercialization of our product candidates; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability to protect the Company's intellectual property; the loss of any executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of regulations that affect the Company's products; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K. Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this release. Except as may be required by law, the Company does not undertake any obligation to release publicly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Matinas BioPharma’s product candidates are all in a development stage and are not available for sale or use . 2

MTNB Overview • Proprietary, NIH - supported, lipid - crystal nano - encapsulation technology • Immediate focus on taking powerful, but sparingly used, anti - fungal and anti - bacterial drugs and increasing safety and potency • MAT2203, an oral formulation of Amphotericin B with key differentiating data, for serious fungal infections, scheduled to report Phase 2a results in 2016 - QIDP and Fast Track designations granted August 2015; positioning for Orphan Drug and Breakthrough Therapy • MAT2501, an oral formulation of Amikacin for severe hospital - acquired bacterial infections scheduled for IND filing in 4Q 2015 • Technology has broad utility with potential in numerous Orphan Drug indications • $10 million financing completed Q2 2015 • Experienced management team and board with track record of building companies 3 Clinical - stage biopharmaceutical company focused on identifying and developing safe and effective antifungal and anti - bacterial therapeutics for the treatment of serious and life - threatening infections
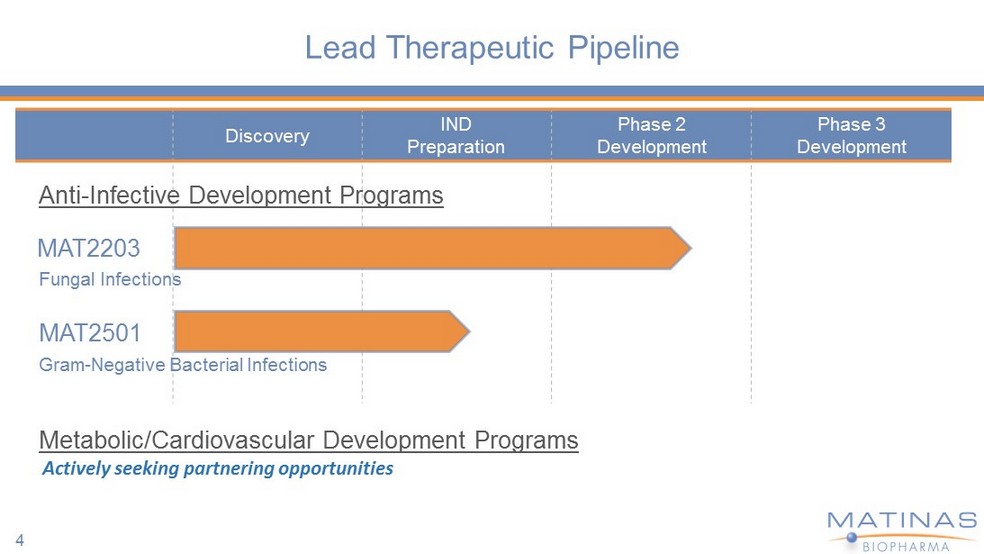
Discovery IND Preparation Phase 2 Development Phase 3 Development Lead Therapeutic Pipeline Gram - Negative Bacterial Infections Metabolic/Cardiovascular Development Programs Anti - Infective Development Programs Fungal Infections MAT2203 MAT2501 4 Actively seeking partnering opportunities

Antimicrobial Resistance is a Global Threat “CDC sets threat levels for drug - resistant 'superbugs’” “Superbugs to kill 'more than cancer' by 2050” “WHO Calls for Action on Superbugs” “CDC sounds alarm on deadly, untreatable superbugs” 5
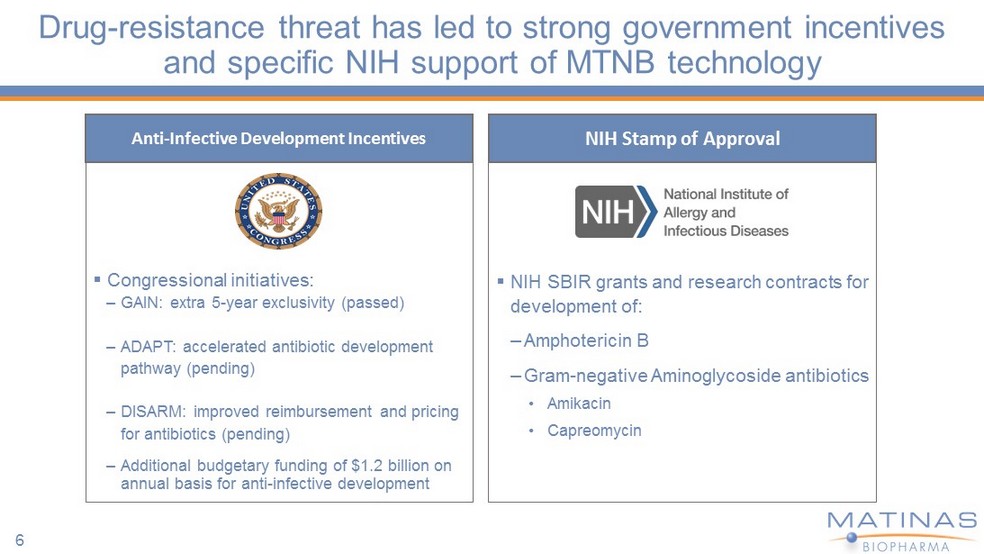
Drug - resistance threat has led to strong government incentives and specific NIH support of MTNB technology ▪ Congressional initiatives: – GAIN: extra 5 - year exclusivity (passed) – ADAPT: accelerated antibiotic development pathway (pending) – DISARM: improved reimbursement and pricing for antibiotics (pending) – Additional budgetary funding of $1.2 billion on annual basis for anti - infective development Anti - Infective Development Incentives ▪ NIH SBIR grants and research contracts for development of: – Amphotericin B – Gram - negative Aminoglycoside antibiotics • Amikacin • Capreomycin NIH Stamp of Approval 6
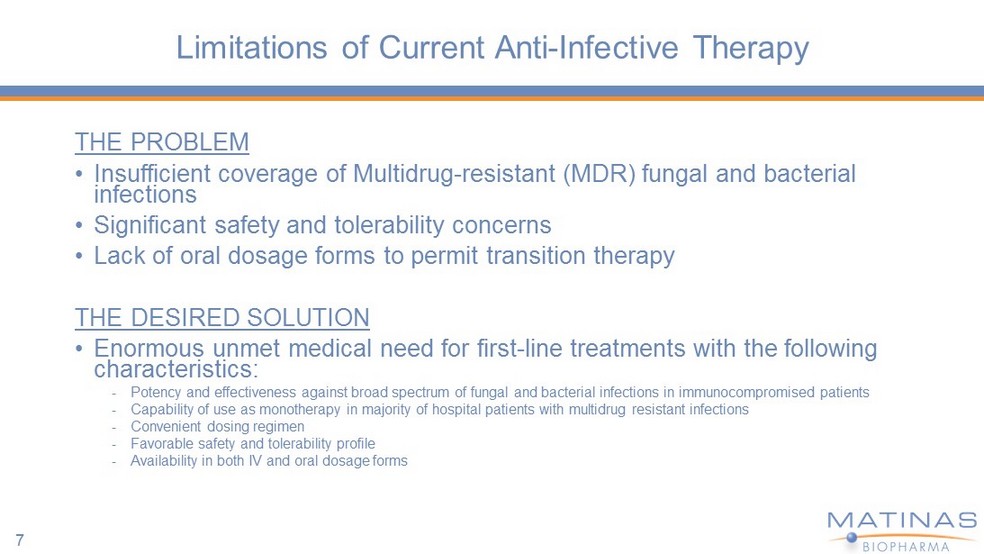
Limitations of Current Anti - Infective Therapy THE PROBLEM • Insufficient coverage of Multidrug - resistant (MDR) fungal and bacterial infections • Significant safety and tolerability concerns • Lack of oral dosage forms to permit transition therapy THE DESIRED SOLUTION • Enormous unmet medical need for first - line treatments with the following characteristics: - Potency and effectiveness against broad spectrum of fungal and bacterial infections in immunocompromised patients - Capability of use as monotherapy in majority of hospital patients with multidrug resistant infections - Convenient dosing regimen - Favorable safety and tolerability profile - Availability in both IV and oral dosage forms 7

Cochleate Technology Offers Significant Clinical Improvement Potential Development Work To Date • Efficacy demonstrated in - vivo (numerous animal studies) • Safety and tolerability demonstrated in Phase 1 human study Protects Organs • Cochleates act as a shield for the body from toxic drugs, significantly reducing adverse effects Targeted Delivery • Cochleates are carried directly to infection sites where payload released, increasing potency Oral Administration • Convenience; health economic benefit vs. IV - therapy in hospital 8
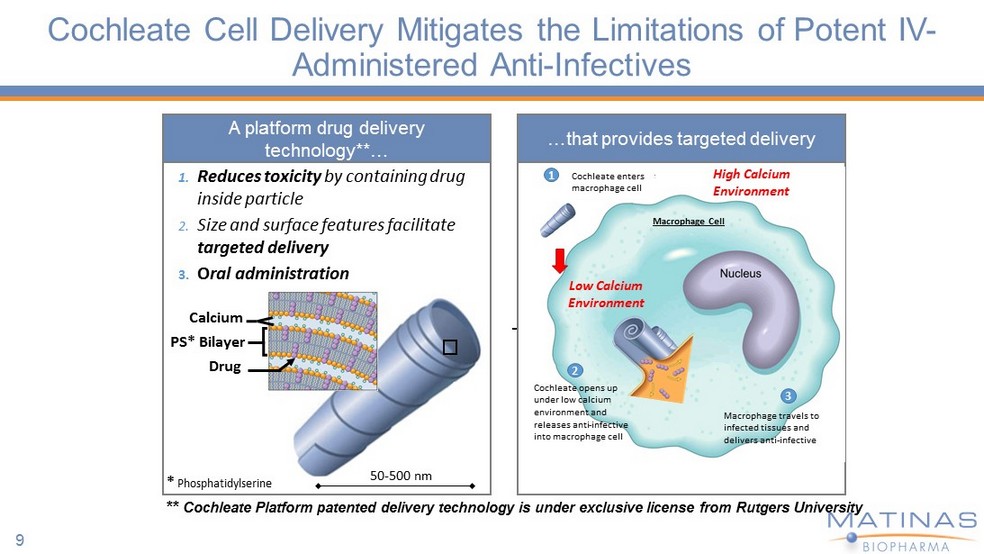
Cochleate Cell Delivery Mitigates the Limitations of Potent IV - Administered Anti - Infectives 50 - 500 nm * Phosphatidylserine PS* Bilayer Calcium Drug 1. Reduces toxicity by containing drug inside particle 2. Size and surface features facilitate targeted delivery 3. O ral administration Low Calcium Environment 1 2 A platform drug delivery technology**… …that provides targeted delivery ** Cochleate Platform patented delivery technology is under exclusive license from Rutgers University Cochleate opens up under low calcium environment and releases anti - infective into macrophage cell 3 Macrophage travels to infected tissues and delivers anti - infective Macrophage Cell Cochleate enters macrophage cell High Calcium Environment 9

MAT2203 Amphotericin B Delivered in a Lipid - Crystal Nano - Particle Cochleate Formulation -- Broad - Spectrum Fungicidal Agent -- 10

MAT2203 Represents Groundbreaking Advancement in Anti - Fungal Treatment • Amphotericin B is the antifungal of choice for immunocompromised patients • Pluses: Effective killing - agent (fungicidal), no observed clinical resistance in 50+ years of use. • Minuses: Significant toxicity, including high risk of liver and kidney damage • Despite limits on use, global market is ~$700 million • Matinas ’ oral formulation has significant advantages, including significantly improved tissue penetration profile, over current IV - only administration of Amphotericin B • Demonstrated efficacy and little - to - no kidney toxicity in animal models as compared to current Amphotericin B therapy • Differentiation supports potential to capture and expand $ 700MM global Amphotericin B market • Granted QIDP and Fast Track designations in August 2015 • Development program to focus on indications with potential for Orphan Drug and Breakthrough Therapy designations • MAT2203 commencing Phase 2a with NIH; data expected 2016 11
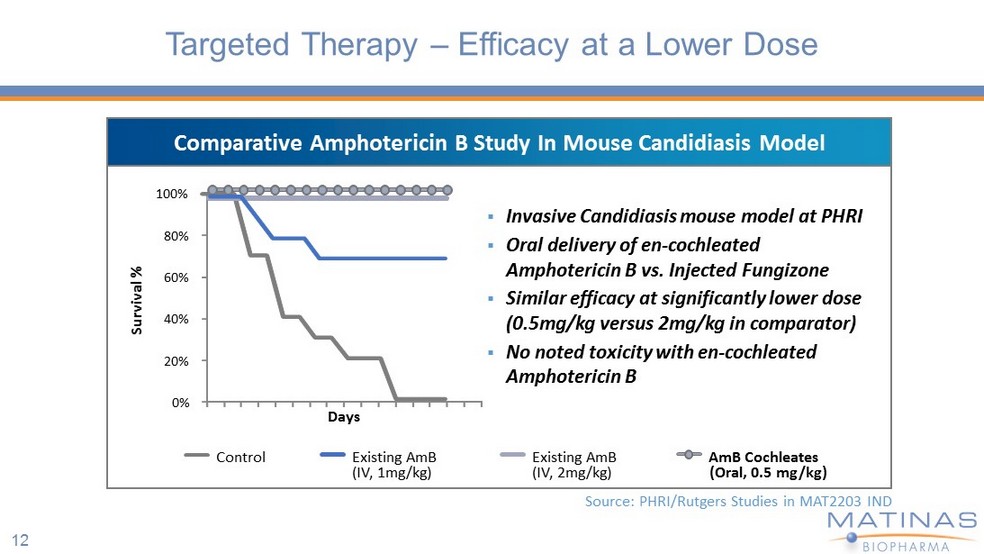
Control Existing AmB (IV, 1mg/kg) Existing AmB (IV, 2mg/kg) AmB Cochleates (Oral, 0.5 mg/kg ) Source: PHRI/Rutgers Studies in MAT2203 IND 12 Targeted Therapy – Efficacy at a Lower Dose Survival % Days 0% 20% 40% 60% 80% 100% ▪ Invasive Candidiasis mouse model at PHRI ▪ Oral delivery of en - cochleated Amphotericin B vs. Injected Fungizone ▪ Similar efficacy at significantly lower dose (0.5mg/kg versus 2mg/kg in comparator) ▪ No noted toxicity with en - cochleated Amphotericin B Comparative Amphotericin B Study In Mouse Candidiasis Model
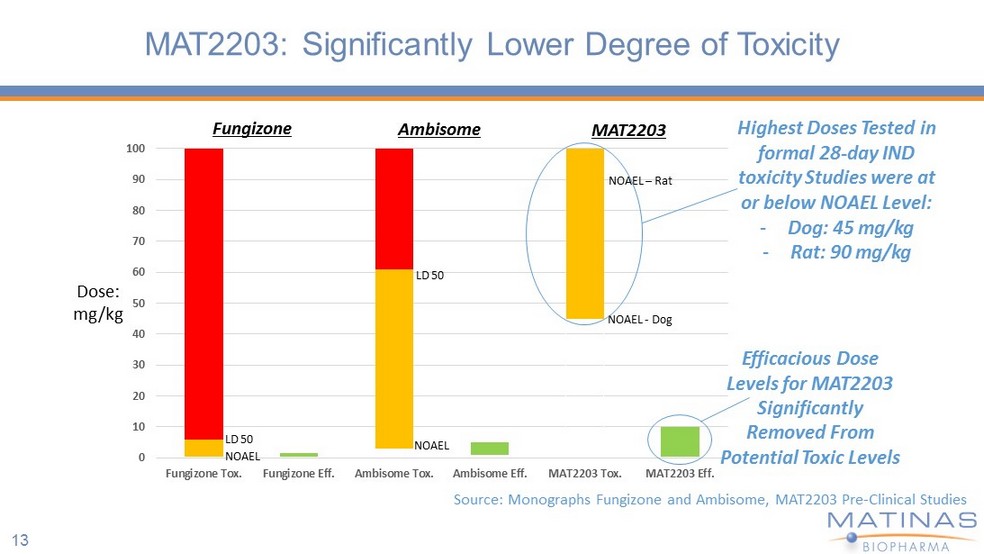
13 MAT2203: Significantly Lower Degree of Toxicity 0 10 20 30 40 50 60 70 80 90 100 Fungizone Tox. Fungizone Eff. Ambisome Tox. Ambisome Eff. MAT2203 Tox. MAT2203 Eff. Fungizone NOAEL - Dog NOAEL – Rat Dose: mg/kg Ambisome MAT2203 Source: Monographs Fungizone and Ambisome , MAT2203 Pre - Clinical Studies Highest Doses Tested in formal 28 - day IND toxicity Studies were at or below NOAEL Level: - Dog: 45 mg/kg - Rat: 90 mg/kg Efficacious Dose Levels for MAT2203 Significantly Removed From Potential Toxic Levels LD 50 NOAEL NOAEL LD 50
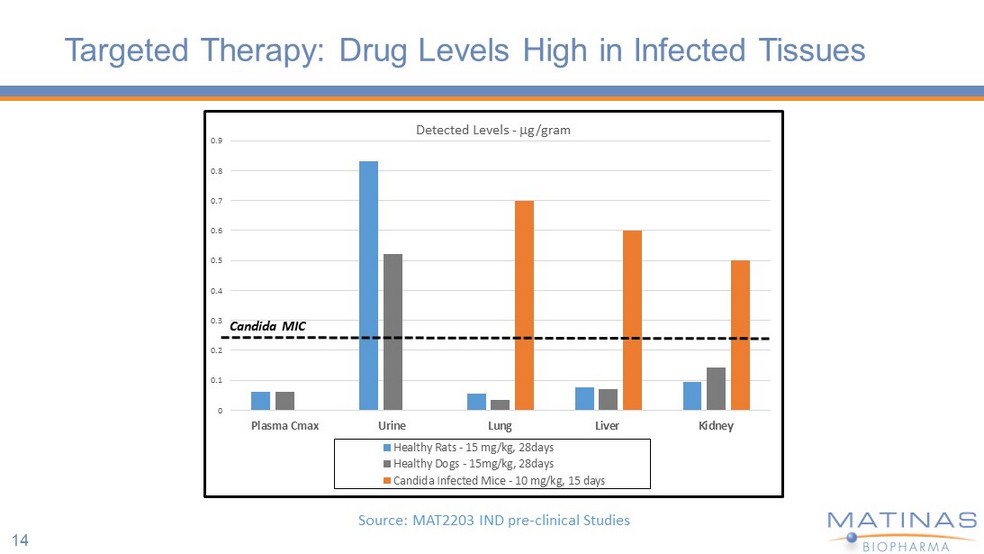
14 Targeted Therapy: Drug Levels High in Infected Tissues 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 Plasma Cmax Urine Lung Liver Kidney Detected Levels - g/gram Healthy Rats - 15 mg/kg, 28days Healthy Dogs - 15mg/kg, 28days Candida Infected Mice - 10 mg/kg, 15 days Candida MIC Source: MAT2203 IND pre - clinical Studies
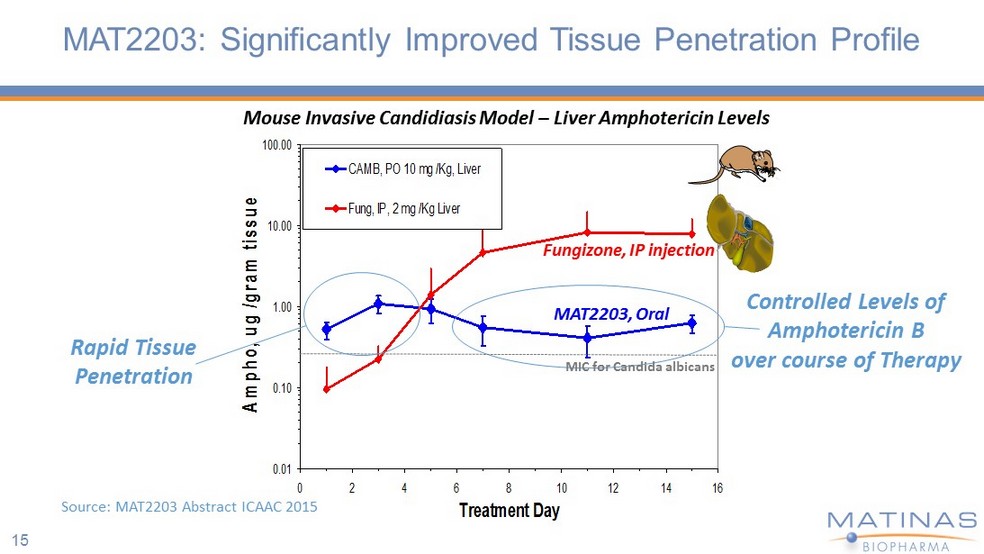
15 MAT2203: Significantly Improved Tissue Penetration Profile 0.01 0.10 1.00 10.00 100.00 0 2 4 6 8 10 12 14 16 Treatment Day Ampho, ug /gram tissue CAMB, PO 10 mg /Kg, Liver Fung, IP, 2 mg /Kg Liver MAT2203, Oral Fungizone , IP injection Rapid Tissue Penetration Controlled Levels of Amphotericin B o ver course of Therapy Mouse Invasive Candidiasis Model – Liver Amphotericin Levels Source: MAT2203 Abstract ICAAC 2015 MIC for Candida albicans

MAT2203 – Clinical Development Overview Discovery IND Preparation Phase 2 Development Phase 3 Development MAT2203 x Successfully completed a range of efficacy animal studies at NIH with C - Amphotericin B x Single - Dose Phase 1 study completed with favorable tolerability and no serious adverse events x Increased production of C - Amphotericin B to ~800 doses/batch – semi - commercial scale Next Steps: ▪ Phase 2a patient treatment protocol under final review in collaboration with NIH/NIAID with study commencing in Q42015 and data expected in 2016 ▪ Engage with FDA on development program post - Phase 2a data 16

MAT2501 Amikacin Delivered in a Lipid - Crystal Nano - Particle Cochleate Formulation -- Gram - Negative Aminoglycoside Antibiotic -- 17

The Drug Resistant Antibiotic Market • Widespread use of antibiotics ($41 billion worldwide per IMS) has resulted in rapid increase in bacterial infections that are resistant to multiple antibacterial agents • Gram - negative bacterial infections characterized as #1 unmet medical need by infectious disease specialists • Effective first - line treatment of serious infections requires use of broad spectrum antibiotics with activity against a broad range of bacteria • Many strains of bacteria have mutated over time, developing resistance to existing drugs • According to 2013 CDC report, 2 million people in the U.S. each year acquire serious infections that are resistant to one or more antibiotics 18
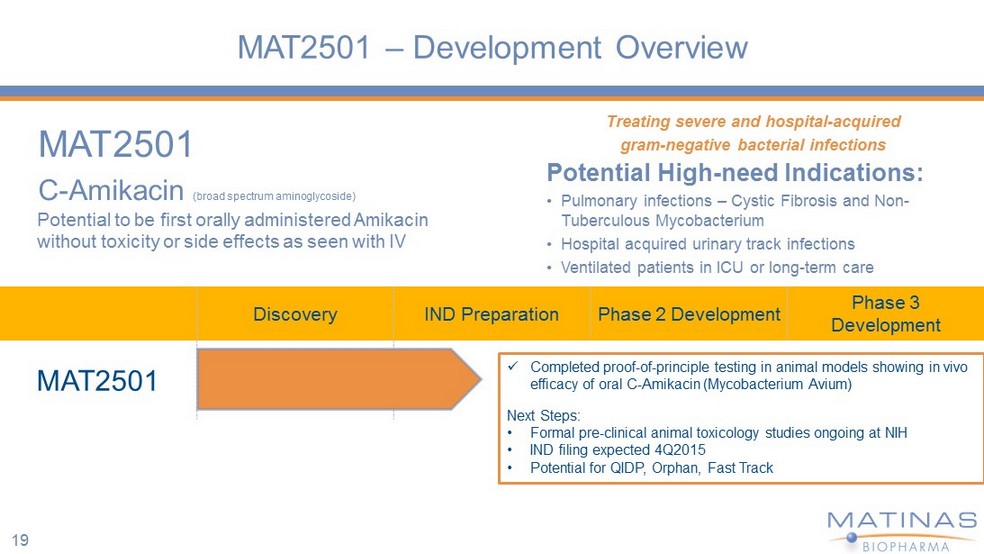
MAT2501 – Development Overview MAT2501 C - Amikacin (broad spectrum aminoglycoside) Treating severe and hospital - acquired gram - negative bacterial infections Potential High - need Indications: • Pulmonary infections – Cystic Fibrosis and Non - Tuberculous Mycobacterium • Hospital acquired urinary track infections • Ventilated patients in ICU or long - term care Discovery IND Preparation Phase 2 Development Phase 3 Development MAT2501 x Completed proof - of - principle testing in animal models showing in vivo efficacy of oral C - Amikacin (Mycobacterium Avium ) Next Steps : • Formal pre - clinical animal t oxicology studies ongoing at NIH • IND filing expected 4Q2015 • Potential for QIDP, Orphan, Fast Track Potential to be first orally administered Amikacin without toxicity or side effects as seen with IV 19
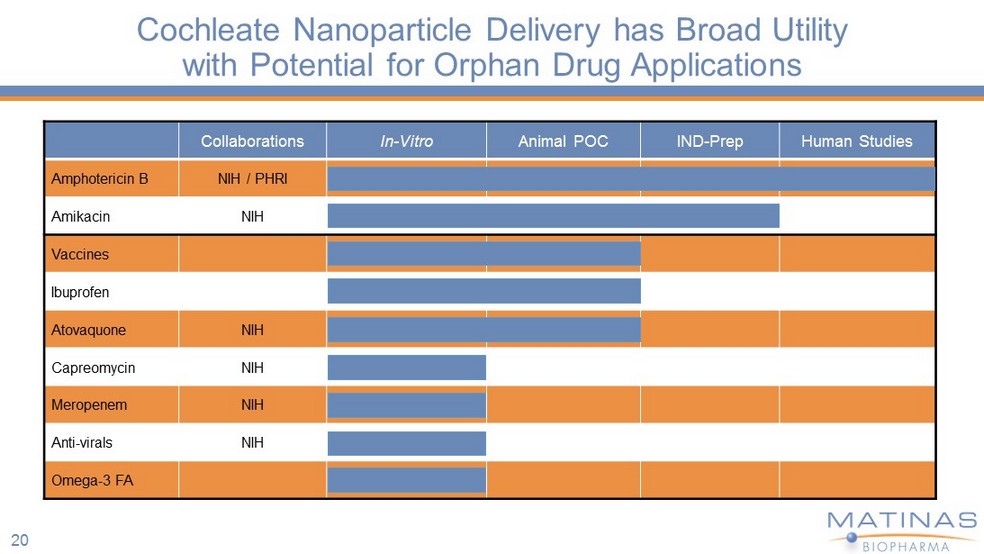
Cochleate Nanoparticle Delivery has Broad Utility with Potential for Orphan Drug Applications Collaborations In - Vitro Animal POC IND - Prep Human Studies Amphotericin B NIH / PHRI Amikacin NIH Vaccines Ibuprofen Atovaquone NIH Capreomycin NIH Meropenem NIH Anti - virals NIH Omega - 3 FA 20

Intellectual Property Cochleate Lipid Delivery Portfolio – Exclusive License from Rutgers University • 17 issued and > 20 pending U.S. and foreign patents - Company controls prosecution - 10 patents issued within past 3 years; Patent protection currently extends through 2027 - Pending applications can extend patent protection through 2033 • Potential for significant regulatory exclusivity (Orphan; GAIN ) 21

Management Team Strong development and commercialization track record Roelof Rongen Co - Founder, Chief Executive Officer, Director Jerome D. Jabbour , JD Co - Founder, Chief Business Officer & General Counsel Raphael J. Mannino Chief Technical Officer George Bobotas , PhD Co - Founder, Chief Scientific Officer Abdel Fawzy , PhD Co - Founder, EVP, Pharmaceutical & Supply Chain Development Gary Gaglione , CPA Chief Financial Officer, Vice President of Finance Douglas F. Kling SVP, Clinical Development and Project Management 22

Board of Directors Herbert Conrad Chairman of the Board James Scietta Director Adam Stern Director Stefano Ferrari Director 23

Building Prominent Scientific Advisory Board Anti - Infectives J. Carl Craft, MD, Chair - Former Chief Scientific Officer for Medicines for Malaria Venture (MMV) - Former Venture Head at Abbott Laboratories Anti - Infective Development Group David S. Perlin , Ph.D. - Internationally renowned expert in infectious disease, with primary expertise in fungal infections and mechanisms of antifungal drug resistance - Executive Director of the Public Health Research Institute (PHRI) - Professor of Microbiology, Biochemistry and Molecular Genetics at New Jersey Medical School Peter G. Pappas, MD, FACP - Professor of Medicine in the Division of Infectious Diseases and Tinsley Harrison Clinical Scholar at the University of Alabama in Birmingham - Principal Investigator for the Mycoses Study Group 24 Expecting to add additional preeminent anti - fungal/anti - bacterial KOL’s in near term
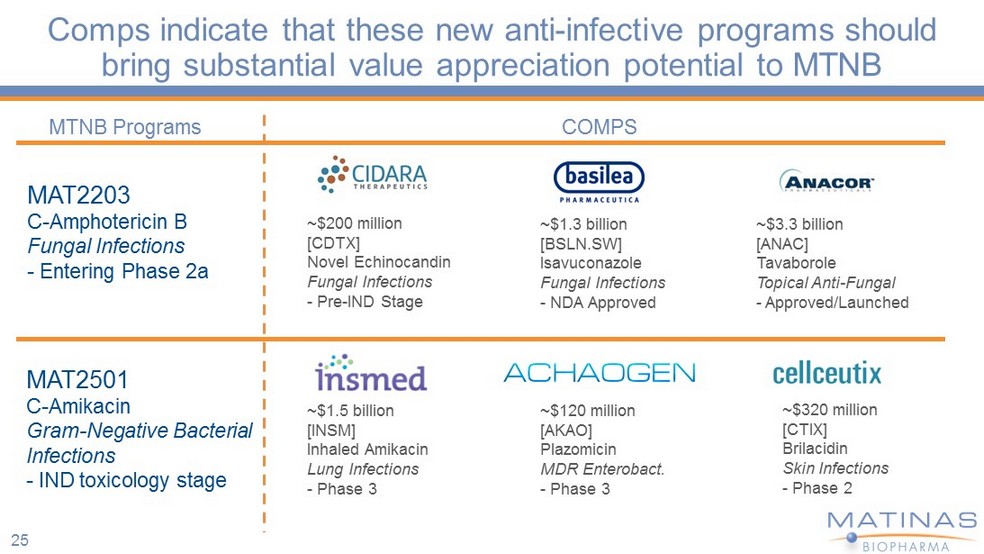
Comps indicate that these new anti - infective programs should bring substantial value appreciation potential to MTNB MAT2203 C - Amphotericin B Fungal Infections - Entering Phase 2a MAT2501 C - Amikacin Gram - Negative Bacterial Infections - IND toxicology stage ~$200 million [CDTX] Novel Echinocandin Fungal Infections - Pre - IND Stage ~$3.3 billion [ANAC] Tavaborole Topical Anti - Fungal - Approved/Launched COMPS MTNB Programs ~$1.3 billion [BSLN.SW] Isavuconazole Fungal Infections - NDA Approved ~$120 m illion [AKAO] Plazomicin MDR Enterobact . - Phase 3 ~$320 million [CTIX] Brilacidin Skin Infections - Phase 2 ~$1.5 billion [INSM] Inhaled Amikacin Lung Infections - Phase 3 25
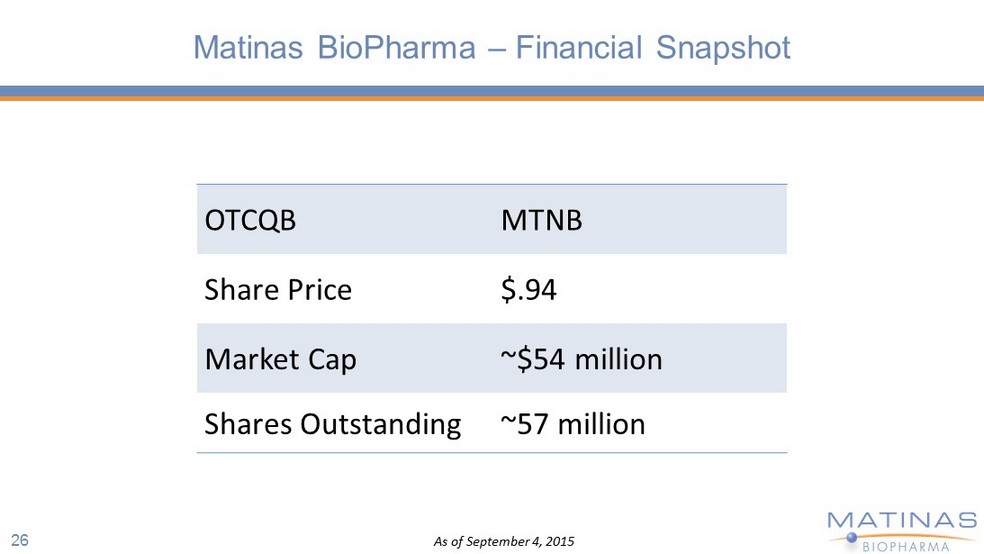
Matinas BioPharma – Financial Snapshot OTCQB MTNB Share Price $.94 Market Cap ~$54 million Shares Outstanding ~57 million As of September 4, 2015 26

MTNB Investment Highlights • Proprietary, NIH - supported lipid - crystal nano - encapsulation technology with true platform potential • Initial focus on taking powerful, but sparingly used, anti - fungal and anti - bacterial drugs and increasing safety and potency • MAT2203, an oral formulation of Amphotericin B with key differentiating data, for serious fungal infections, scheduled to report Phase 2a results in 2016 - QIDP and Fast Track designations granted August 2015; positioning for Orphan Drug and Breakthrough Therapy • MAT2501 , an oral formulation of Amikacin for severe hospital - acquired bacterial infections scheduled for IND filing in 4Q 2015 • $10 million financing completed Q2 2015 • Experienced management team and board with track record of building companies 27 Clinical - stage biopharmaceutical company focused on identifying and developing safe and effective antifungal and anti - bacterial therapeutics for the treatment of serious and life - threatening infections

www.matinasbiopharma.com OTCQB: MTNB TRANSFORMING THE WAY POTENT MEDICINES FOR INFECTIOUS DISEASES ARE DESIGNED Corporate Presentation September 2015