
Exhibit 99.1

www.matinasbiopharma.com OTCQB: MTNB TRANSFORMING THE WAY POTENT MEDICINES FOR INFECTIOUS DISEASES ARE DESIGNED MAT2203 CLINICAL DEVELOPMENT UPDATE CALL OCTOBER 6, 2016

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 19 95, including those relating to the Company's strategic focus and the future development of its product candidates, including MAT 220 3, the anticipated timing of regulatory submissions, the anticipated timing of clinical studies, the Company’s ability to identi fy and pursue development and partnership opportunities for its products or platform delivery technology on favorable terms, if at a ll, and the ability to obtain required regulatory approval and other statements that are predictive in nature, that depend upon or re fer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - lookin g statements. Forward - looking statements include words such as "expects," "anticipates," "intends," "plans," "could," "believes," "estimates" and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statemen ts. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obt ain additional capital to meet our liquidity needs on acceptable terms, or at all, including the additional capital which will be ne cessary to complete the clinical trials of our product candidates; our ability to successfully complete research and further development an d commercialization of our product candidates; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability to maintain and derive benefit from the Qualified Infectious Disease Product (QID P), Orphan and/or Fast Track designations for MAT2203, which does not change the standards for regulatory approval or guarantee regulatory approval on an expedited basis, or at all; our ability to protect the Company's intellectual property; the loss of an y executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of reg ula tions that affect the Company's products; and the other factors listed under "Risk Factors" in our filings with the SEC, including For ms 10 - K, 10 - Q and 8 - K. Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this presentation. Except as may be required by law, the Company does not undertake any obligation to release pub lic ly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occu rrence of unanticipated events. Matinas BioPharma's product candidates are all in a development stage and are not available for sale or use. 2
Agenda 3 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling - SVP Clinical Development Closing Remarks and Q&A
Agenda 4 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling - SVP Clinical Development Closing Remarks and Q&A
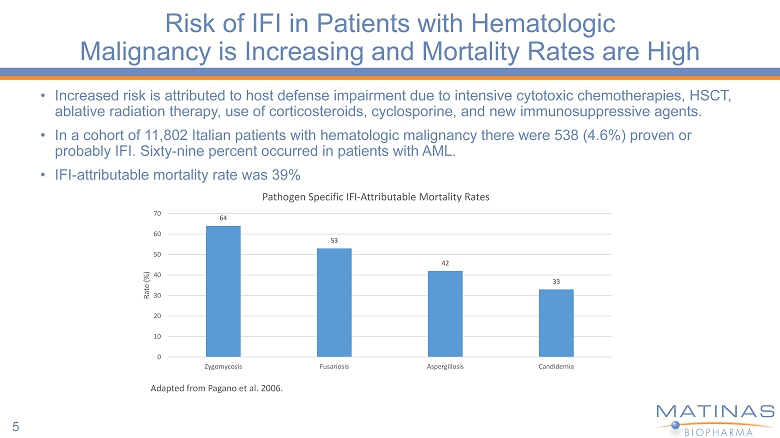
Risk of IFI in Patients with Hematologic Malignancy is Increasing and Mortality Rates are High • Increased risk is attributed to host defense impairment due to intensive cytotoxic chemotherapies, HSCT, ablative radiation therapy, use of corticosteroids, cyclosporine, and new immunosuppressive agents. • In a cohort of 11,802 Italian patients with hematologic malignancy there were 538 (4.6%) proven or probably IFI. Sixty - nine percent occurred in patients with AML. • IFI - attributable mortality rate was 39% 5 64 53 42 33 0 10 20 30 40 50 60 70 Zygomycosis Fusariosis Aspergillosis Candidemia Rate (%) Adapted from Pagano et al. 2006. Pathogen Specific IFI - Attributable Mortality Rates
Agenda 6 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling - SVP Clinical Development Closing Remarks and Q&A
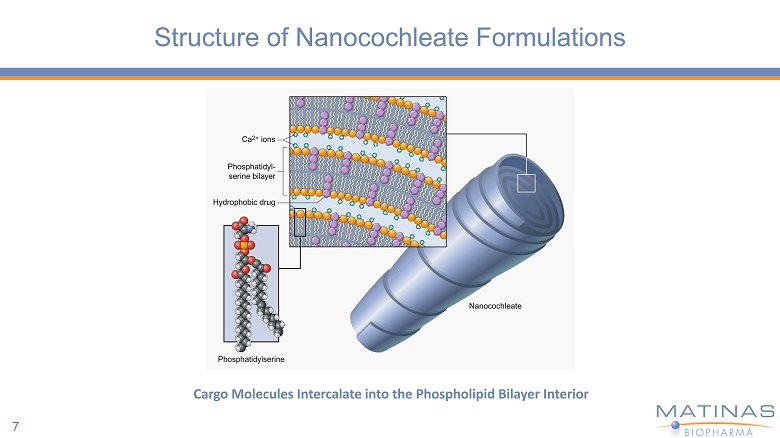
Structure of Nanocochleate Formulations 7 Cargo Molecules Intercalate into the Phospholipid Bilayer Interior
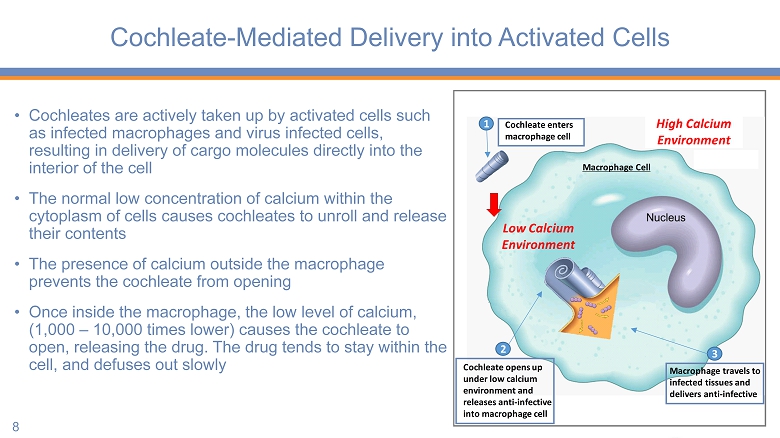
Cochleate - Mediated Delivery into Activated Cells • Cochleates are actively taken up by activated cells such as infected macrophages and virus infected cells, resulting in delivery of cargo molecules directly into the interior of the cell • The normal low concentration of calcium within the cytoplasm of cells causes cochleates to unroll and release their contents • The presence of calcium outside the macrophage prevents the cochleate from opening • Once inside the macrophage, the low level of calcium, (1,000 – 10,000 times lower) causes the cochleate to open, releasing the drug. The drug tends to stay within the cell, and defuses out slowly 8 Low Calcium Environment 1 2 Cochleate opens up under low calcium environment and releases anti - infective into macrophage cell 3 Macrophage travels to infected tissues and delivers anti - infective Macrophage Cell Cochleate enters macrophage cell High Calcium Environment
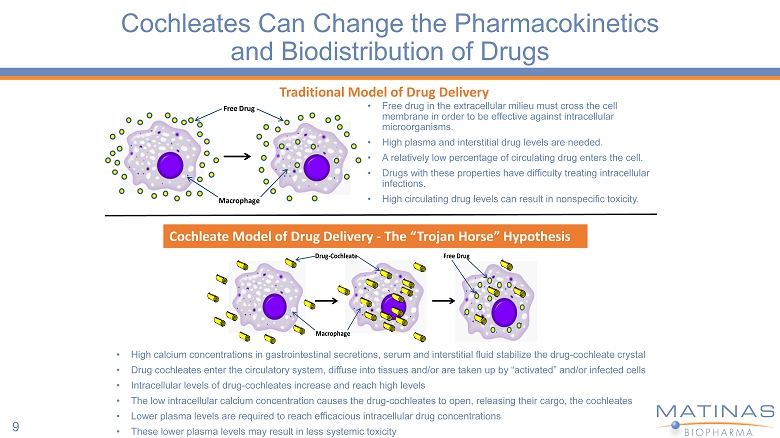
Cochleates Can Change the Pharmacokinetics and Biodistribution of Drugs 9 Macrophage Free Drug • Free drug in the extracellular milieu must cross the cell membrane in order to be effective against intracellular microorganisms. • High plasma and interstitial drug levels are needed. • A relatively low percentage of circulating drug enters the cell. • Drugs with these properties have difficulty treating intracellular infections. • High circulating drug levels can result in nonspecific toxicity. Drug - Cochleate Macrophage Free Drug Cochleate Model of Drug Delivery - The “Trojan Horse” Hypothesis Traditional Model of Drug Delivery • High calcium concentrations in gastrointestinal secretions, serum and interstitial fluid stabilize the drug - cochleate crystal • Drug cochleates enter the circulatory system, diffuse into tissues and/or are taken up by “activated” and/or infected cells • Intracellular levels of drug - cochleates increase and reach high levels • The low intracellular calcium concentration causes the drug - cochleates to open, releasing their cargo, the cochleates • Lower plasma levels are required to reach efficacious intracellular drug concentrations • These lower plasma levels may result in less systemic toxicity
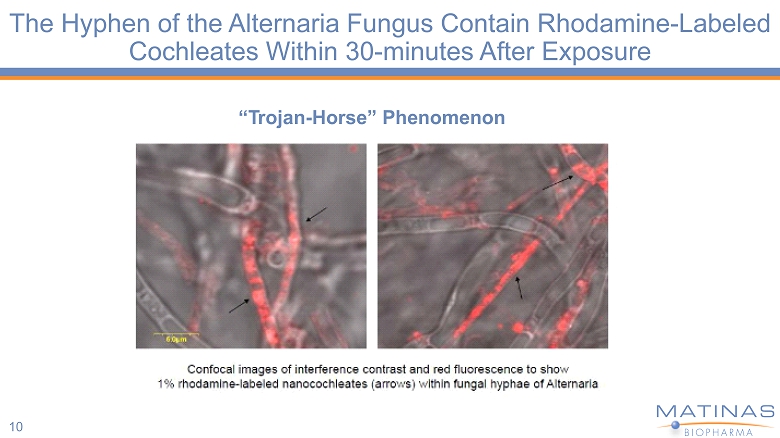
The Hyphen of the Alternaria Fungus Contain Rhodamine - Labeled Cochleates Within 30 - minutes After Exposure 10 “ T rojan - H o rse” Ph e nome n on
Agenda 11 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling - SVP Clinical Development Closing Remarks and Q&A
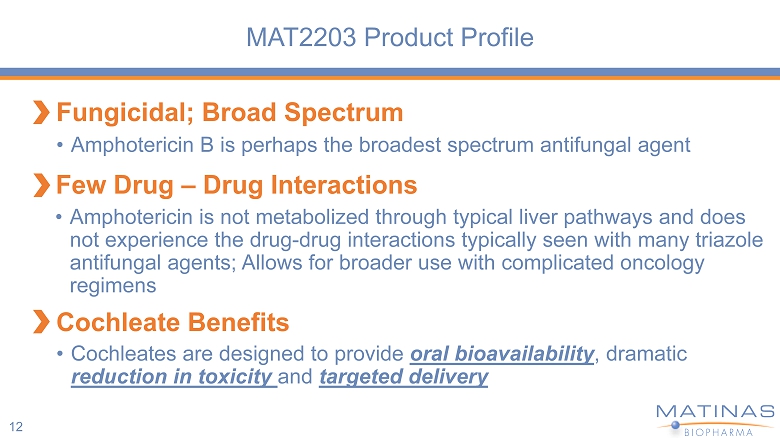
MAT2203 Product Profile 12 Fungicidal; Broad Spectrum Few Drug – Drug Interactions Cochleate Benefits • Amphotericin B is perhaps the broadest spectrum antifungal agent • Amphotericin is not metabolized through typical liver pathways and does not experience the drug - drug interactions typically seen with many triazole antifungal agents; Allows for broader use with complicated oncology regimens • Cochleates are designed to provide oral bioavailability , dramatic reduction in toxicity and targeted delivery
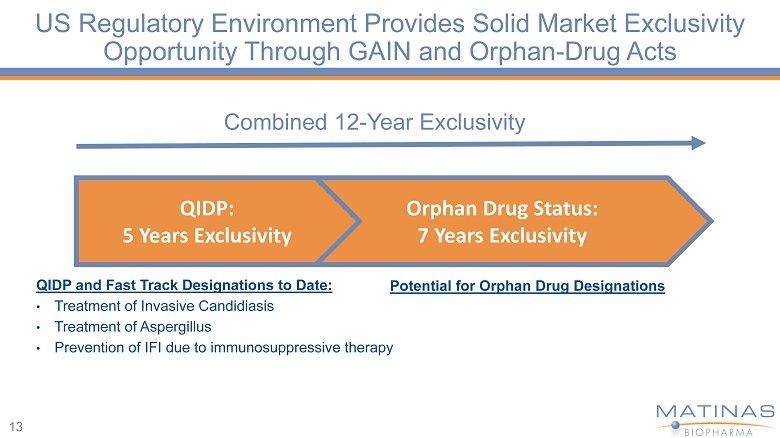
13 Combined 12 - Year Exclusivity QIDP and Fast Track Designations to Date: • Treatment of Invasive Candidiasis • Treatment of Aspergillus • Prevention of IFI due to immunosuppressive therapy Orphan Drug Status: 7 Years Exclusivity QIDP: 5 Years Exclusivity US Regulatory Environment Provides Solid Market Exclusivity Opportunity Through GAIN and Orphan - Drug Acts Potential for Orphan Drug Designations
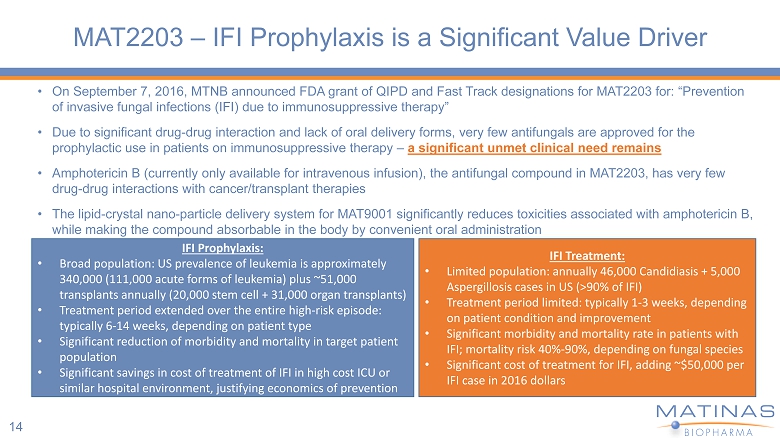
MAT2203 – IFI Prophylaxis is a Significant Value Driver • On September 7, 2016, MTNB announced FDA grant of QIPD and Fast Track designations for MAT2203 for: “Prevention of invasive fungal infections (IFI) due to immunosuppressive therapy” • Due to significant drug - drug interaction and lack of oral delivery forms, very few antifungals are approved for the prophylactic use in patients on immunosuppressive therapy – a significant unmet clinical need remains • Amphotericin B (currently only available for intravenous infusion), the antifungal compound in MAT2203, has very few drug - drug interactions with cancer/transplant therapies • The lipid - crystal nano - particle delivery system for MAT9001 significantly reduces toxicities associated with amphotericin B, while making the compound absorbable in the body by convenient oral administration IFI Prophylaxis: • Broad population: US prevalence of leukemia is approximately 340,000 (111,000 acute forms of leukemia) plus ~51,000 transplants annually (20,000 stem cell + 31,000 organ transplants) • Treatment period extended over the entire high - risk episode: typically 6 - 14 weeks, depending on patient type • Significant reduction of morbidity and mortality in target patient population • Significant savings in cost of treatment of IFI in high cost ICU or similar hospital environment, justifying economics of prevention IFI Treatment: • Limited population: annually 46,000 Candidiasis + 5,000 Aspergillosis cases in US (>90% of IFI) • Treatment period limited: typically 1 - 3 weeks, depending on patient condition and improvement • Significant morbidity and mortality rate in patients with IFI; mortality risk 40% - 90%, depending on fungal species • Significant cost of treatment for IFI, adding ~$50,000 per IFI case in 2016 dollars 14
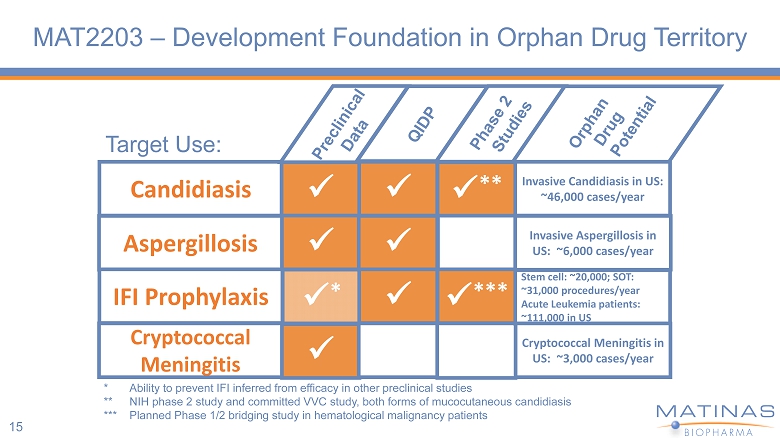
MAT2203 – Development Foundation in Orphan Drug Territory 15 Target Use: Candidiasis Aspergillosis IFI Prophylaxis Cryptococcal Meningitis x x x * x x x x x ** x *** Invasive Candidiasis in US: ~46,000 cases/year Invasive Aspergillosis in US: ~6,000 cases/year Stem cell: ~20,000; SOT: ~31,000 procedures/year Acute Leukemia patients: ~111,000 in US Cryptococcal Meningitis in US: ~3,000 cases/year * Ability to prevent IFI inferred from efficacy in other preclinical studies ** NIH phase 2 study and committed VVC study, both forms of mucocutaneous candidiasis *** Planned Phase 1/2 bridging study in hematological malignancy patients
Agenda 16 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling SVP Clinical Development Closing Remarks and Q&A
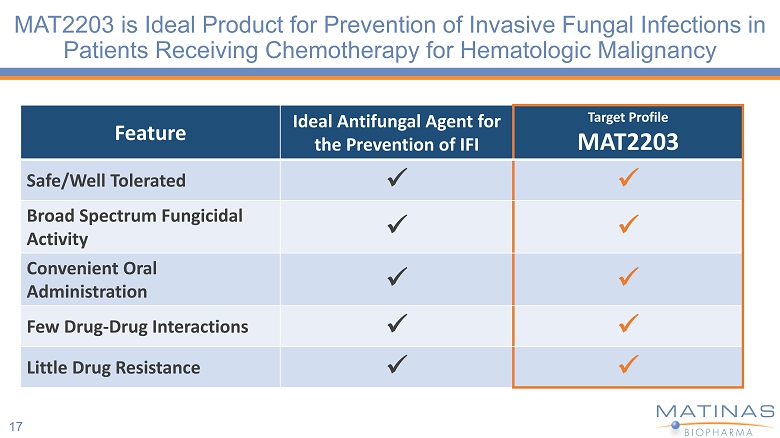
MAT2203 is Ideal Product for Prevention of Invasive Fungal Infections in Patients Receiving Chemotherapy for Hematologic Malignancy 17 Feature Ideal Antifungal Agent for the Prevention of IFI Target Profile MAT2203 Safe/Well Tolerated x x Broad Spectrum Fungicidal Activity x x Convenient Oral Administration x x Few Drug - Drug Interactions x x Little Drug Resistance x x
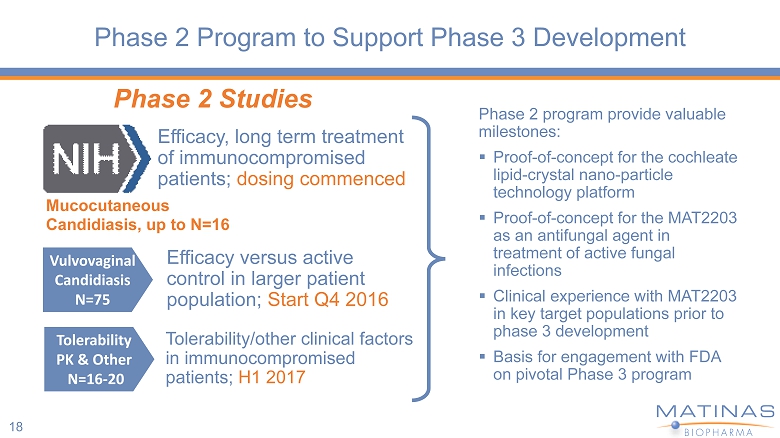
Phase 2 Program to Support Phase 3 Development 18 Phase 2 program provide valuable milestones: ▪ Proof - of - concept for the cochleate lipid - crystal nano - particle technology platform ▪ Proof - of - concept for the MAT2203 as an antifungal agent in treatment of active fungal infections ▪ Clinical experience with MAT2203 in key target populations prior to phase 3 development ▪ Basis for engagement with FDA on pivotal Phase 3 program Mucocutaneous Candidiasis, up to N=16 Phase 2 Studies Efficacy, long term treatment of immunocompromised patients; dosing commenced Vulvovaginal Candidiasis N=75 Tolerability PK & Other N=16 - 20 Efficacy versus active control in larger patient population; Start Q4 2016 Tolerability/other clinical factors in immunocompromised patients; H1 2017

Ongoing NIAID/Matinas Phase 2a Trial in Patients with Chronic Mucocutaneous Candidiasis • Goal: Evaluate antifungal efficacy and safety with orally administered CAMB • Patients: Patients with diagnosis of persistent oropharyngeal (OPC), esophageal (EC), or vulvovaginal candidiasis (VVC) who are refractory or intolerant to standard non - intravenous therapies (N=16) • Primary Objective : To assess the clinical response to treatment of mucocutaneous candidiasis (OPC, EC, VVC) infections after treatment for 14 - days with the highest titrated dosage (Target Dosage) of CAMB per patient. • Secondary Objectives : • To assess the plasma, urine, and salivary PK of AMB after a single dose and after multiple doses (14 days) • To assess the safety and tolerability • To assess mycological response Clinicaltrials.gov: NCT02629419 19

Phase 2 Trial in Vulvovaginal Candidiasis (VVC) • Goal: Evaluate antifungal safety and efficacy with orally administered CAMB • Patients: Women with diagnosis of moderate to severe VVC (N=75) • Primary Objective: To evaluate the safety of oral CAMB 200 mg/day for 4 days • Secondary Objectives: - Clinical cure rate - Mycology eradication - Responder outcome (clinical + mycology) • Tertiary Objective: Multiple dose PK in CAMB arm • Control Arm: Fluconazole 150 mg (single - dose) 20
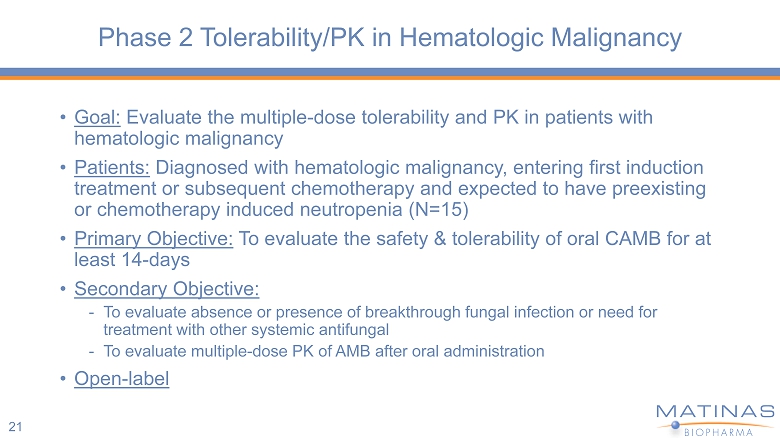
Phase 2 Tolerability/PK in Hematologic Malignancy • Goal: Evaluate the multiple - dose tolerability and PK in patients with hematologic malignancy • Patients: Diagnosed with hematologic malignancy, entering first induction treatment or subsequent chemotherapy and expected to have preexisting or chemotherapy induced neutropenia (N=15) • Primary Objective: To evaluate the safety & tolerability of oral CAMB for at least 14 - days • Secondary Objective: - To evaluate absence or presence of breakthrough fungal infection or need for treatment with other systemic antifungal - To evaluate multiple - dose PK of AMB after oral administration • Open - label 21

Phase 3 Development Plans • Phase 3 study for the prevention of IFI in patients diagnosed with hematologic malignancy, entering first induction treatment or subsequent chemotherapy and expected to have preexisting or chemotherapy induced neutropenia - N = 400 - 500 patients - Double - blind control group - Primary endpoint: Incidence of IFI - Secondary endpoint: Safety 22
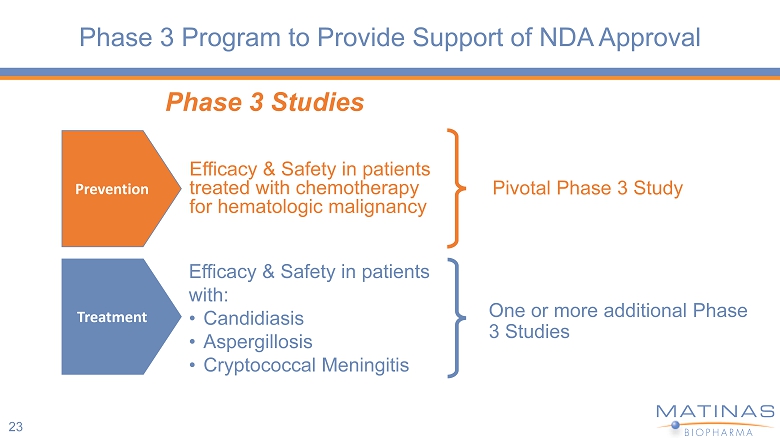
Phase 3 Program to Provide Support of NDA Approval 23 Pivotal Phase 3 Study Phase 3 Studies Efficacy & Safety in patients treated with chemotherapy for hematologic malignancy Prevention Treatment Efficacy & Safety in patients with: • Candidiasis • Aspergillosis • Cryptococcal Meningitis One or more additional Phase 3 Studies
Agenda 24 Forward Looking Statements / Introduction Jerry Jabbour President Peter Pappas, MD - UAB and President MSG Raphael Mannino, Ph.D., Chief Technology Officer Roelof Rongen CEO Douglas Kling - SVP Clinical Development Closing Remarks and Q&A

www.matinasbiopharma.com OTCQB: MTNB TRANSFORMING THE WAY POTENT MEDICINES FOR INFECTIOUS DISEASES ARE DESIGNED MAT2203 CLINICAL DEVELOPMENT UPDATE CALL OCTOBER 6, 2016