
Exhibit 99.2

www.matinasbiopharma.com NYSE MKT: MTNB Quarterly Update Conference Call and Webcast April 3, 2017

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - looking statements. Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could,” “believes,” “estimates” and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or at all, including the additional capital which will be necessary to complete the clinical trials of our product candidates; our ability to successfully complete research and further development and commercialization of our product candidates; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability to protect the Company's intellectual property; the loss of any executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of regulations that affect the Company's products; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K. Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this release. Except as may be required by law, the Company does not undertake any obligation to release publicly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Matinas BioPharma’s product candidates are all in a development stage and are not available for sale or use. 2

Jerome D. Jabbour President 3

Recent Highlights Set the stage for a transformational 2017 Anti - Infective Development Program Achievements - Launched Phase 2 development program for lead anti - infective candidate, MAT2203, an orally - administered, encochleated formulation of the broad spectrum fungicidal medication amphotericin B - Commenced patient dosing in the NIH sponsored Phase 2a clinical study for the treatment of refractory mucocutaneous candidiasis infection - Announced the Institutional Review Board of the NIAID, part of NIH granted approval for a 6 - month open - label safety extension of the Phase 2a study - Commenced patient dosing in Phase 2 clinical study in patients with vulvovaginal candidiasis - Launched Phase 1 development program of MAT2501, an orally - administered formulation of the broad spectrum IV - only aminoglycoside antibiotic agent amikacin - Announced positive topline results of Phase 1 study of MAT2501 in healthy volunteers Recent Corporate Highlights - Received Notice of Allowance of U.S. Patent for novel lipid - crystal nano - particle cochleate formulation technology - Secured a GLP lab space/ GMP commercial scale manufacturing facility - Successfully completed warrant tender offer with gross proceeds of $13.5 million from exercise of warrants - Commenced trading on the NYSE MKT - Bolstered board of directors with the appoint of Eric J. Ende, MBA, MD 4
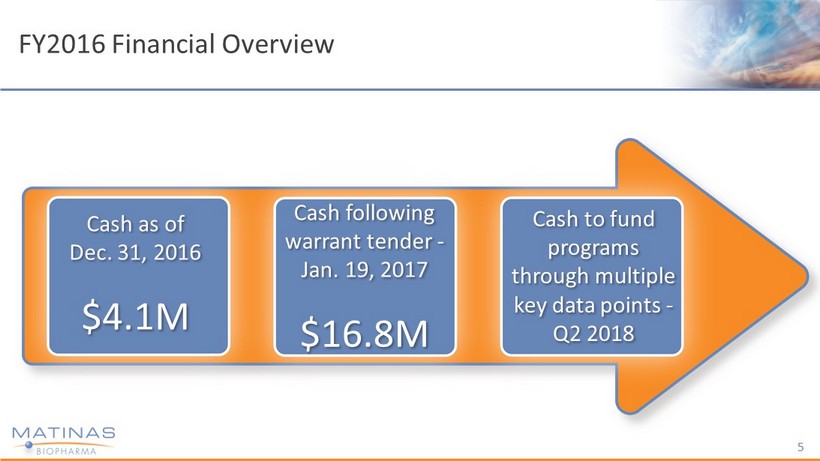
FY2016 Financial Overview 5 Cash following warrant tender - Jan. 19, 2017 $16.8M Cash to fund programs through multiple key data points - Q2 2018 Cash as of Dec. 31, 2016 $4.1M

Roelof Rongen Chief Executive Officer 6

MAT2501 Targeting NTM and Drug - Resistant Gram - Negative Bacterial Infections • MAT2501 formulates the broad - spectrum aminoglycoside Amikacin into our lipid - crystal nano - particle cochleate technology • Active IND for the treatment of Non - Tuberculous - Mycobacterium (NTM) infections (environmentally transmitted organisms) - Chronic lung infection with similar course of progression as Tuberculosis - Approximately 50,000 to 90,000 patients in the US; 40% refractory to treatment - IV amikacin used as add - on therapy in refractory patients • Demonstrated efficacy in pre - clinical models of disseminated NTM and pulmonary NTM infections, as well as biofilm models of NTM • MAT2501 received QIDP and Orphan Drug designations from FDA • Company is exploring treatment of more acute drug - resistant gram negative infections 7

MAT2501 Positive Phase 1 Results • Single - ascending - dose study in healthy volunteers (200, 400, 800mg) • Evaluated drug kinetics (blood, urine, feces), tolerability and acute safety • Peak blood levels were an area of focus; IV amikacin is know to generate high peak levels with potential kidney - and neuro - toxic effects (hearing loss), therefore safety limits exist for IV amikacin (<10 µg/ml before redosing, peak levels not to exceed 35 µg/ml) - Single - dose MAT2501 Amikacin peak levels did not exceed 0.1 µg/ml, leaving more than 100 x safety margin; our thesis: this is supportive of meeting this parameter under multi - dosing - Other kinetic data indicating significant absorption and distribution: e.g. single - dose peak levels exceeding 3 µg/ml in urine (30 - 40x blood level), >0.2 µg/ml on day 2 (>2x peak plasma) • No serious adverse (AE) events reported - Most Adverse Events were of mild an gastro - intestinal nature - These AEs were similar to those seen with MAT2203; not believed of antibiotic nature • Tolerability data appear to support 400mg BID dosing; to be confirmed in multiple - ascending dose Phase 1 study (evaluate: kinetics, safety, tolerability) 8
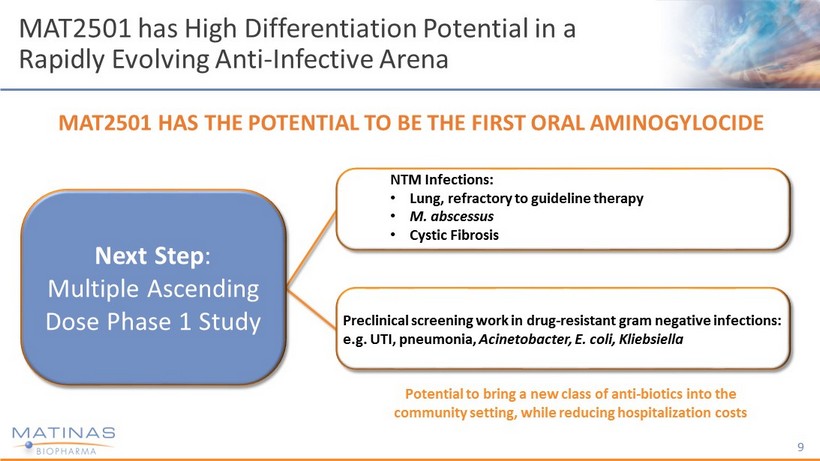
9 MAT2501 has High Differentiation Potential in a Rapidly Evolving Anti - Infective Arena Preclinical screening work in drug - resistant gram negative infections: e.g. UTI, pneumonia, Acinetobacter, E. coli, Kliebsiella NTM Infections: • Lung, refractory to guideline therapy • M. abscessus • Cystic Fibrosis Next Step : Multiple Ascending Dose Phase 1 Study MAT2501 HAS THE POTENTIAL TO BE THE FIRST ORAL AMINOGYLOCIDE Potential to bring a new class of anti - biotics into the community setting, while reducing hospitalization costs
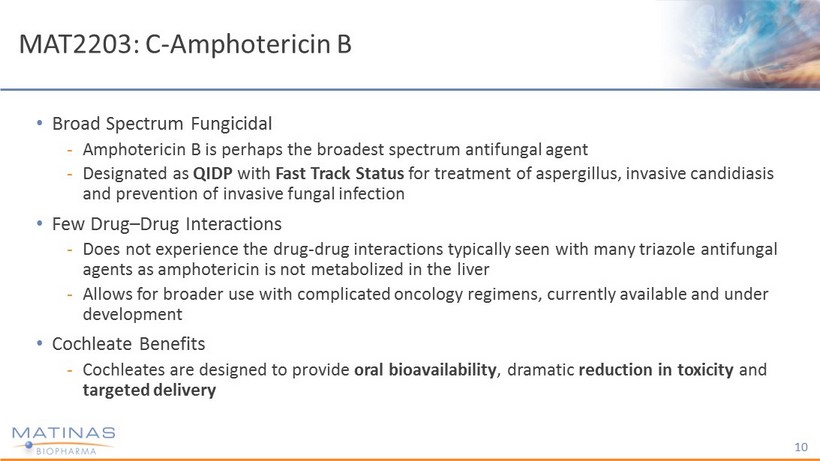
MAT2203: C - Amphotericin B • Broad Spectrum Fungicidal - Amphotericin B is perhaps the broadest spectrum antifungal agent - Designated as QIDP with Fast Track Status for treatment of aspergillus, invasive candidiasis and prevention of invasive fungal infection • Few Drug – Drug Interactions - Does not experience the drug - drug interactions typically seen with many triazole antifungal agents as amphotericin is not metabolized in the liver - Allows for broader use with complicated oncology regimens, currently available and under development • Cochleate Benefits - Cochleates are designed to provide oral bioavailability , dramatic reduction in toxicity and targeted delivery 10
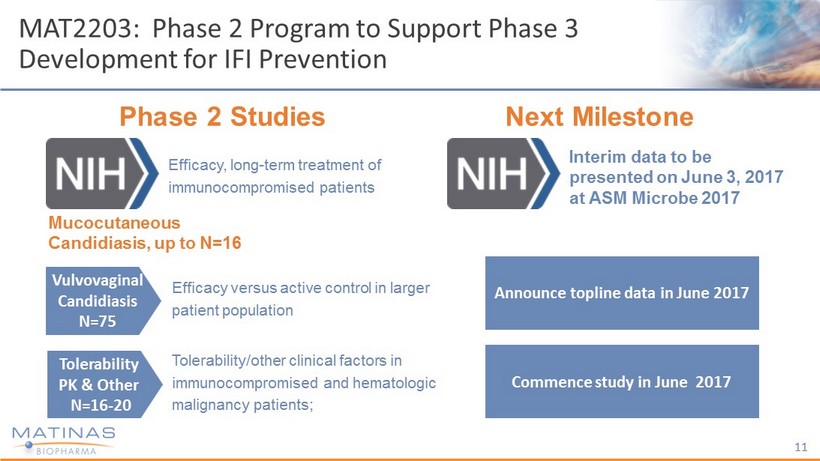
MAT2203: Phase 2 Program to Support Phase 3 Development for IFI Prevention Mucocutaneous Candidiasis, up to N=16 Phase 2 Studies Vulvovaginal Candidiasis N=75 Tolerability PK & Other N=16 - 20 Efficacy versus active control in larger patient population Tolerability/other clinical factors in immunocompromised and hematologic malignancy patients; 11 Next Milestone Interim data to be presented on June 3, 2017 at ASM Microbe 2017 Announce topline data in June 2017 Commence study in June 2017 Efficacy, long - term treatment of immunocompromised patients

Q&A 12

www.matinasbiopharma.com NYSE MKT: MTNB Quarterly Update Conference Call and Webcast April 3, 2017