
Exhibit 99.1

www.matinasbiopharma.com NYSE MKT: MTNB Canaccord Genuity 37 th Annual Growth Conference Jerrome D. Jabbour, Co - founder and President

Forward Looking Statement This presentation contains "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 , including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions . All statements other than statements of historical fact are statements that could be forward - looking statements . Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could,” “believes,” “estimates” and similar expressions . These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements . Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or at all, including the additional capital which will be necessary to complete the clinical trials of our product candidates ; our ability to successfully complete research and further development and commercialization of our product candidates ; the uncertainties inherent in clinical testing ; the timing, cost and uncertainty of obtaining regulatory approvals ; our ability to protect the Company's intellectual property ; the loss of any executive officers or key personnel or consultants ; competition ; changes in the regulatory landscape or the imposition of regulations that affect the Company's products ; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K . Investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this release . Except as may be required by law, the Company does not undertake any obligation to release publicly any revisions to such forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events . Matinas BioPharma’s product candidates are all in a development stage and are not available for sale or use . 2
Overview • Clinical - stage biopharmaceutical company focused on developing innovative anti - infectives for orphan indications • Flagship product, MAT2203 – orally - administered, encochleated amphotericin B, a broad spectrum fungicidal agent preparing to enter Phase 3 - Commencing tolerability/PK study in Q4 2017 in leukemia patients, with data expected in mid - 2018 - Engaging FDA Q4 2017 with the goal to enter Phase 3 registration trial in prevention of invasive fungal infections (IFI) in patients with Acute Lymphoblastic Leukemia (ALL) as quickly as possible • MAT2501 – orally - administered, encochleated amikacin, a broad spectrum aminoglycoside antibiotic agent – initially targeting NTM indication - Phase 1 multiple ascending dose PK study to commence Q4 2017 • Strategic opportunities leveraging cochleate technology to improve therapeutic profile of drugs for potential partners 3
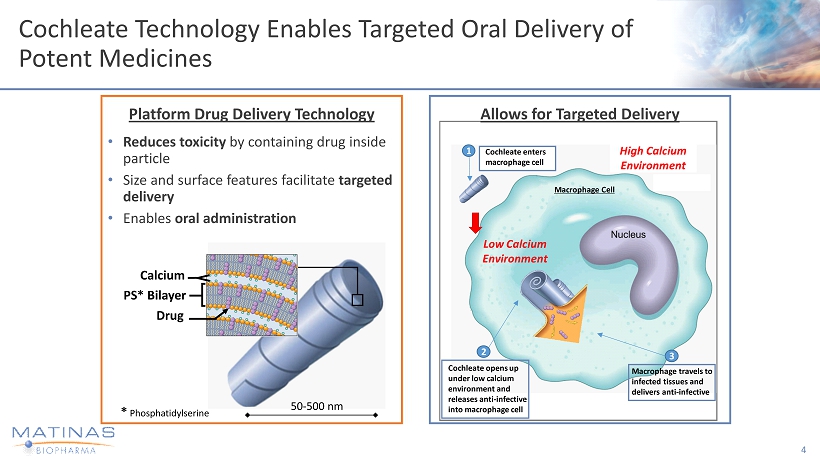
Cochleate Technology Enables Targeted Oral Delivery of Potent Medicines Low Calcium Environment 1 2 Cochleate opens up under low calcium environment and releases anti - infective into macrophage cell 3 Macrophage travels to infected tissues and delivers anti - infective Macrophage Cell Cochleate enters macrophage cell High Calcium Environment Platform Drug Delivery Technology • Reduces toxicity by containing drug inside particle • Size and surface features facilitate targeted delivery • Enables oral administration 50 - 500 nm * Phosphatidylserine PS* Bilayer Calcium Drug Allows for Targeted Delivery 4
MAT2203: C - Amphotericin B ( CAmB ) › Broad Spectrum Fungicidal - Amphotericin B is perhaps the broadest spectrum antifungal agent - Designated as QIDP with Fast Track Status for treatment of aspergillus, invasive candidiasis and prevention of invasive fungal infections › Few Drug – Drug Interactions - Does not experience the drug - drug interactions typically seen with many triazole antifungal agents - Allows for broader use with complicated oncology regimens › Cochleate Benefits - Cochleates are designed to provide oral bioavailability , dramatic reduction in toxicity and targeted delivery 5
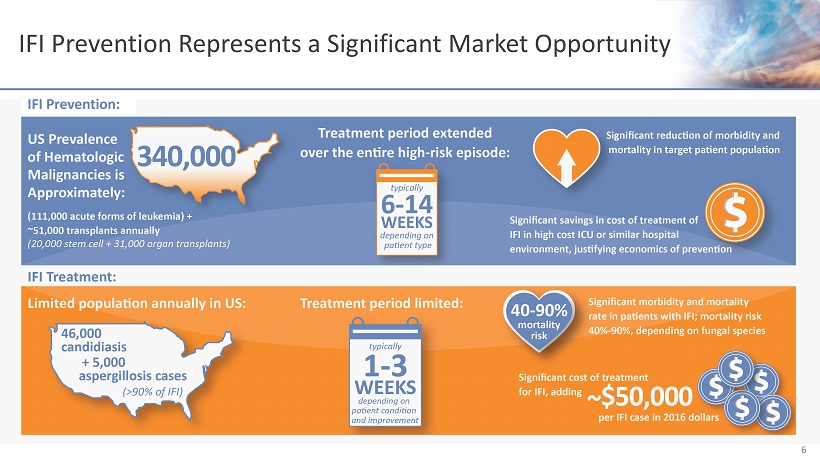
IFI Prevention Represents a Significant Market Opportunity 6 IFI Prevention:
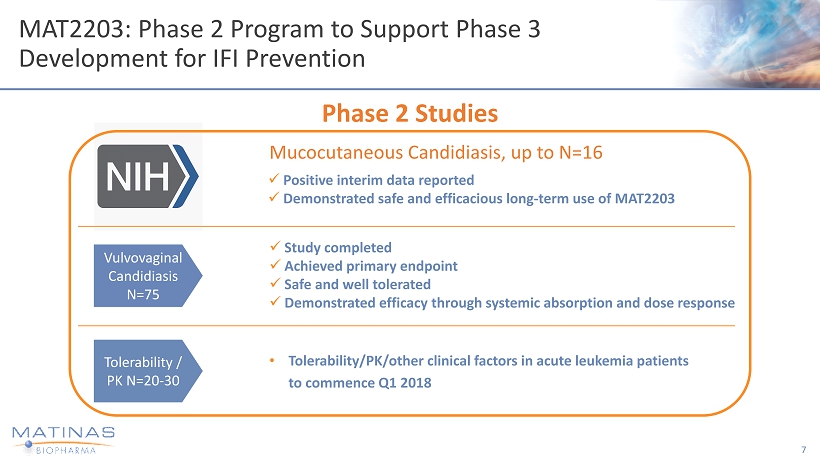
MAT2203: Phase 2 Program to Support Phase 3 Development for IFI Prevention 7 Phase 2 Studies x Positive interim data reported x Demonstrated safe and efficacious long - term use of MAT2203 Mucocutaneous Candidiasis, up to N=16 Vulvovaginal Candidiasis N=75 x Study completed x Achieved primary endpoint x Safe and well tolerated x Demonstrated efficacy through systemic absorption and dose response Tolerability / PK N=20 - 30 • Tolerability/PK/other clinical factors in acute leukemia patients to commence Q1 2018

MAT2203 – Interim Data from NIH Mucocutaneous Candidiasis Phase 2 Study • Two out of two patients met the primary endpoint in achieving ≥ 50% clinical response • Patients reported meaningful Quality of Life improvements - Able to eat a greater variety of foods, including those that are acidic and spicy - Reported less pain • Objective evidence of effect were also observed, decreased severity of lesions, decreased quantitative fungal cultures • No serious adverse events reported to date during the course of the study • MAT2203 was well tolerated with majority of adverse events observed to date being mild in severity and unrelated to MAT2203 • IRB - approved two separate six - month extensions; first two patients elected to enroll and both have now been under treatment for more than eight months 8
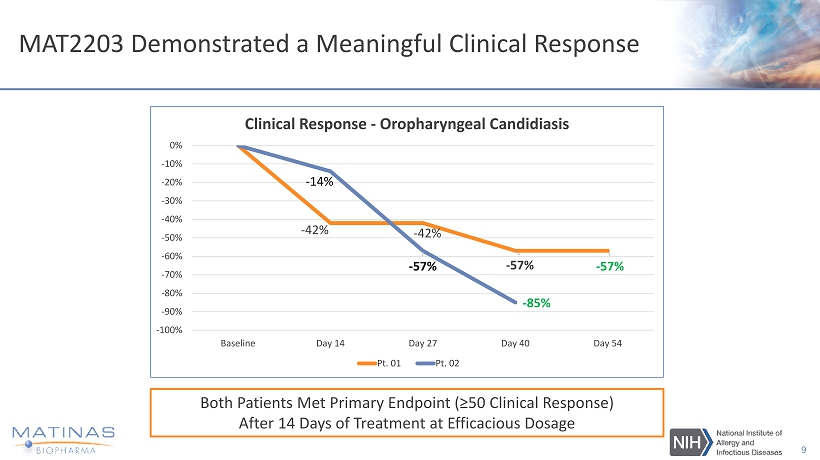
MAT2203 Demonstrated a Meaningful Clinical Response 9 - 42% - 42% - 57% - 57% - 14% - 57% - 85% -100% -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% Baseline Day 14 Day 27 Day 40 Day 54 Clinical Response - Oropharyngeal Candidiasis Pt. 01 Pt. 02 Both Patients Met Primary Endpoint (≥50 Clinical Response) After 14 Days of Treatment at Efficacious Dosage
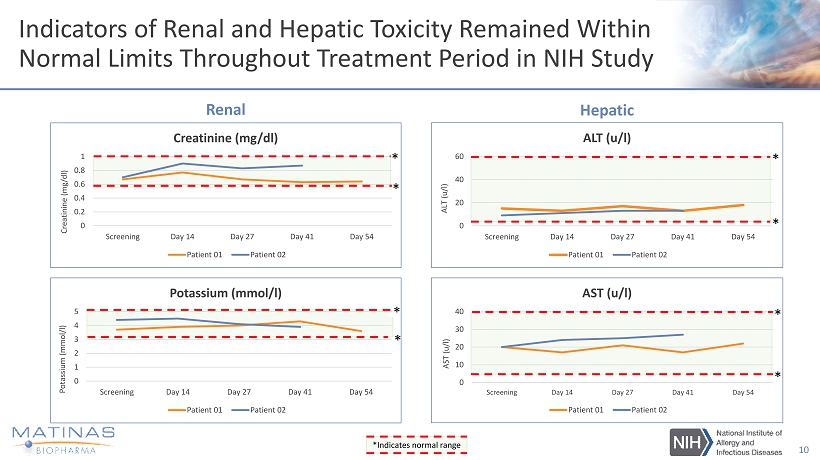
Indicators of Renal and Hepatic Toxicity Remained Within Normal Limits Throughout Treatment Period in NIH Study 10 * * * * * * * * 0 0.2 0.4 0.6 0.8 1 Screening Day 14 Day 27 Day 41 Day 54 Creatinine (mg/dl) Creatinine (mg/dl) Patient 01 Patient 02 0 1 2 3 4 5 Screening Day 14 Day 27 Day 41 Day 54 Potassium (mmol/l) Potassium ( mmol /l) Patient 01 Patient 02 Renal 0 20 40 60 Screening Day 14 Day 27 Day 41 Day 54 ALT (u/l) ALT (u/l) Patient 01 Patient 02 * * Hepatic 0 10 20 30 40 Screening Day 14 Day 27 Day 41 Day 54 AST (u/l) AST (u/l) Patient 01 Patient 02 * * *Indicates normal range
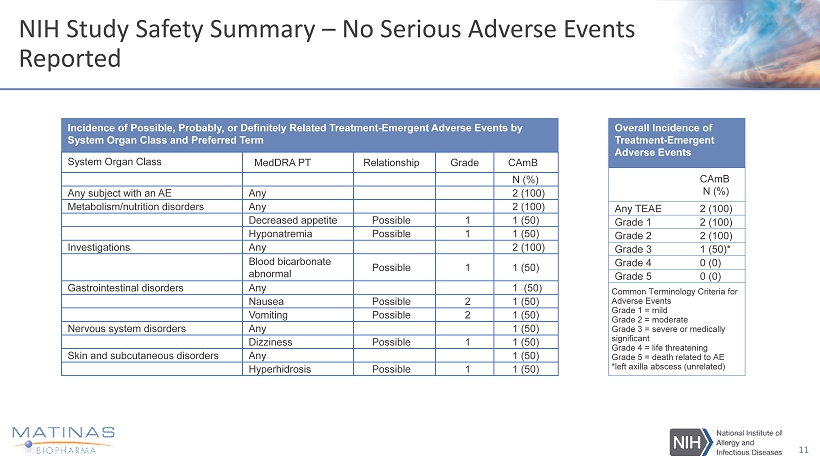
NIH Study Safety Summary – No Serious Adverse Events Reported 11 Incidence of Possible, Probably, or Definitely Related Treatment - Emergent Adverse Events by System Organ Class and Preferred Term System Organ Class MedDRA PT Relationship Grade CAmB N (%) Any subject with an AE Any 2 (100) Metabolism/nutrition disorders Any 2 (100) Decreased appetite Possible 1 1 (50) Hyponatremia Possible 1 1 (50) Investigations Any 2 (100) Blood bicarbonate abnormal Possible 1 1 (50) Gastrointestinal disorders Any 1 (50) Nausea Possible 2 1 (50) Vomiting Possible 2 1 (50) Nervous system disorders Any 1 (50) Dizziness Possible 1 1 (50) Skin and subcutaneous disorders Any 1 (50) Hyperhidrosis Possible 1 1 (50) Overall Incidence of Treatment - Emergent Adverse Events CAmB N (%) Any TEAE 2 (100) Grade 1 2 (100) Grade 2 2 (100) Grade 3 1 (50)* Grade 4 0 (0) Grade 5 0 (0) Common Terminology Criteria for Adverse Events Grade 1 = mild Grade 2 = moderate Grade 3 = severe or medically significant Grade 4 = life threatening Grade 5 = death related to AE *left axilla abscess (unrelated)
Takeaways from Vulvovaginal Candidiasis (VVC) Phase 2 Study • VVC study chosen to further establish the safety and tolerability profile of MAT2203 while demonstrating efficacy through a mechanism involving systemic absorption • VVC provided increased patient exposure to expedite path toward a pivotal registration trial • Study of MAT2203 in patients with primary VVC infections: - Substantial reduction of severity attributes (~ 80% reduction over 12 days) - Clinical cure rates >50% with dose - response observed for 400mg versus 200mg MAT2203 dose 12
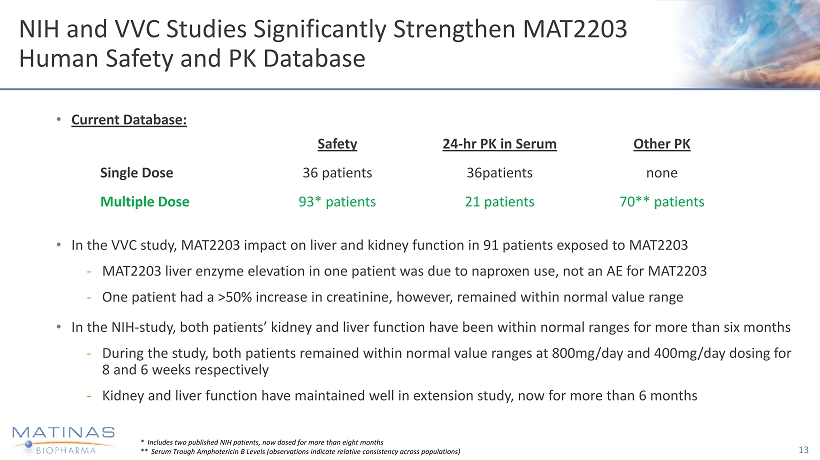
NIH and VVC Studies Significantly Strengthen MAT2203 Human Safety and PK Database • Current Database: • In the VVC study, MAT2203 impact on liver and kidney function in 91 patients exposed to MAT2203 - MAT2203 liver enzyme elevation in one patient was due to naproxen use, not an AE for MAT2203 - One patient had a >50% increase in creatinine, however, remained within normal value range • In the NIH - study, both patients’ kidney and liver function have been within normal ranges for more than six months - During the study, both patients remained within normal value ranges at 800mg/day and 400mg/day dosing for 8 and 6 weeks respectively - Kidney and liver function have maintained well in extension study, now for more than 6 months 13 * Includes two published NIH patients, now dosed for more than eight months ** Serum Trough Amphotericin B Levels (observations indicate relative consistency across populations) Safety 24 - hr PK in Serum Other PK Single Dose 36 patients 36patients none Multiple Dose 93* patients 21 patients 70** patients
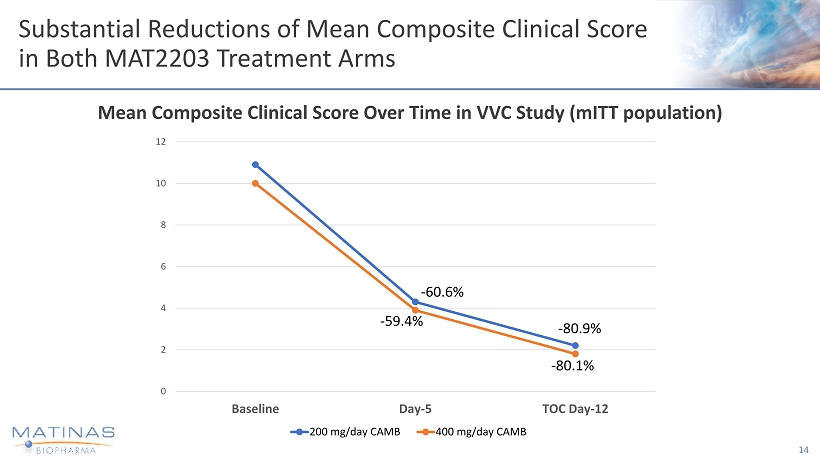
Substantial Reductions of Mean Composite Clinical Score in Both MAT2203 Treatment Arms 14 Mean Composite Clinical Score Over Time in VVC Study ( mITT population) 0 2 4 6 8 10 12 Baseline Day-5 TOC Day-12 200 mg/day CAMB 400 mg/day CAMB - 60.6% - 80.9% - 59.4% - 80.1%
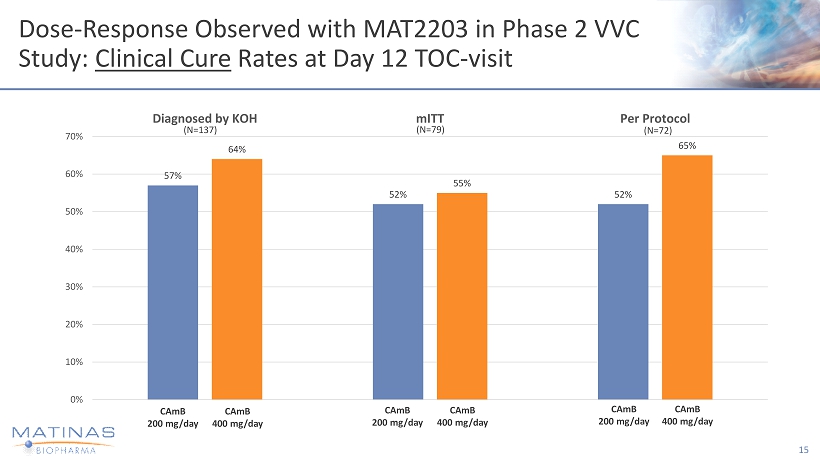
Dose - Response Observed with MAT2203 in Phase 2 VVC Study: Cl i nical C ure Ra t es at Day 12 TOC - visit 15 CA m B 2 0 0 m g /d a y CA m B 2 0 0 m g /d a y CA m B 4 0 0 m g /d a y CA m B 40 0 m g / d a y 57% 52% 52% 64% 55% 65% 0% 10% 20% 30% 40% 50% 60% 70% Diagnosed by KOH mITT Per Protocol CA m B 2 0 0 m g /d a y CA m B 4 0 0 m g /d a y (N=79) (N=72) (N=137)
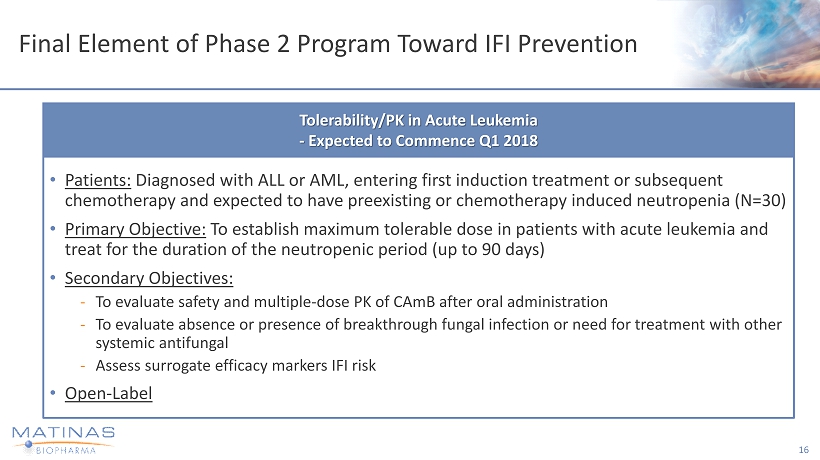
Final Element of Phase 2 Program Toward IFI Prevention • Patients: Diagnosed with ALL or AML, entering first induction treatment or subsequent chemotherapy and expected to have preexisting or chemotherapy induced neutropenia (N=30) • Primary Objective: To establish maximum tolerable dose in patients with acute leukemia and treat for the duration of the neutropenic period (up to 90 days) • Secondary Objectives: - To evaluate safety and multiple - dose PK of CAmB after oral administration - To evaluate absence or presence of breakthrough fungal infection or need for treatment with other systemic antifungal - Assess surrogate efficacy markers IFI risk • Open - Label 16 Tolerability/PK in Acute Leukemia - Expected to Commence Q1 2018
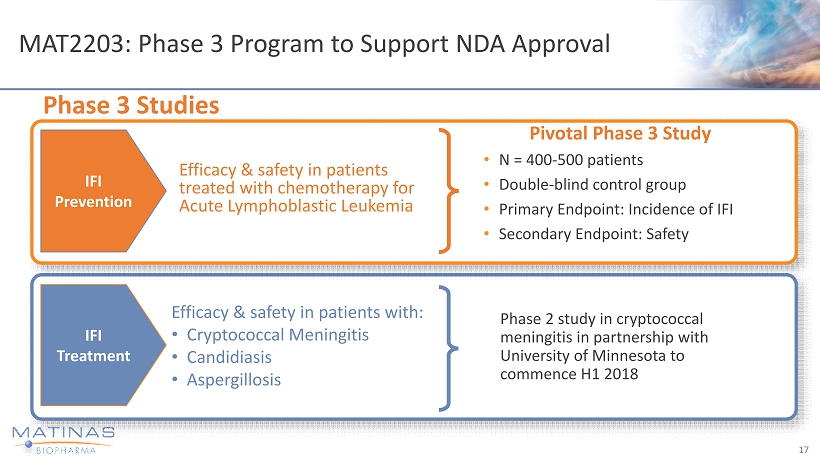
MAT2203: Phase 3 Program to Support NDA Approval 17 Pivotal Phase 3 Study • N = 400 - 500 patients • Double - blind control group • Primary Endpoint: Incidence of IFI • Secondary Endpoint: Safety Phase 3 Studies Efficacy & safety in patients treated with chemotherapy for Acute Lymphoblastic Leukemia IFI Prevention IFI Treatment Efficacy & safety in patients with: • Cryptococcal Meningitis • Candidiasis • Aspergillosis Phase 2 study in cryptococcal meningitis in partnership with University of Minnesota to commence H1 2018
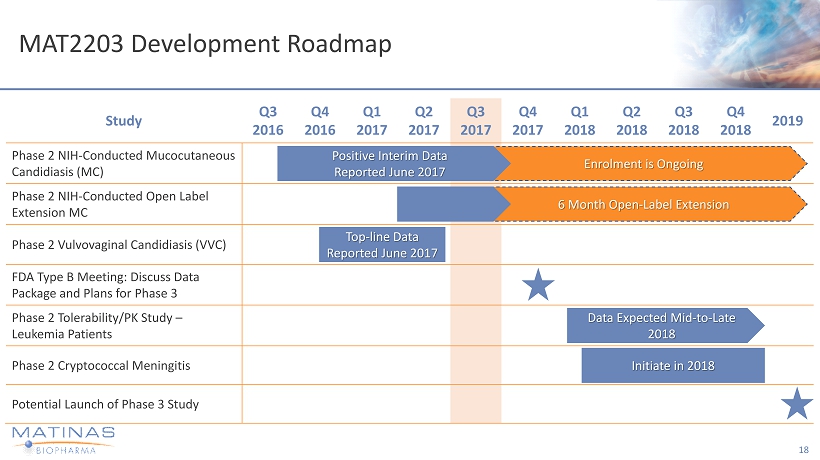
MAT2203 Development Roadmap 18 Study Q3 2016 Q4 2016 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 Q3 2018 Q4 2018 2019 Phase 2 NIH - Conducted Mucocutaneous Candidiasis (MC) Phase 2 NIH - Conducted Open Label Extension MC Phase 2 Vulvovaginal Candidiasis (VVC) FDA Type B Meeting: Discuss Data Package and Plans for Phase 3 Phase 2 Tolerability/PK Study – Leukemia Patients Phase 2 Cryptococcal Meningitis Potential Launch of Phase 3 Study Enrolment is Ongoing Positive Interim Data Reported June 2017 6 Month Open - Label Extension Top - line Data Reported June 2017 Data Expected Mid - to - Late 2018 Initiate in 2018

MAT2501: C - Amikacin › Broad Spectrum Aminoglycoside - Designated as QIDP and received Orphan Drug Designation for treatment of non - tuberculous mycobacterium (NTM) - Positive Phase 1 reported March 2017 › Potential Additional High - Need Indications - Cystic fibrosis associated lung infections - Drug - resistant urinary track infections - Pneumonia in Hospital/ICU or long - term care › Cochleate Benefits - Potential to be first orally administered amikacin without toxicity or side effects as seen with IV 19

MAT2501 Targeting NTM and Drug - Resistant Gram - Negative Bacterial Infections • MAT2501 formulates the broad - spectrum aminoglycoside amikacin into our lipid - crystal nano - particle cochleate technology • Active IND for the treatment of non - tuberculous mycobacterium (NTM) infections (environmentally transmitted organisms) - Chronic lung infection with similar course of progression as tuberculosis - Approximately 50,000 to 90,000 patients in the US; 40% refractory to treatment - IV amikacin used as add - on therapy in refractory patients • Demonstrated efficacy in preclinical models of disseminated NTM and pulmonary NTM infections, as well as biofilm models of NTM • Company is exploring treatment of more acute drug - resistant gram - negative infections 20

MAT2501 Positive Phase 1 Results • Single - ascending dose study in healthy volunteers (200, 400, 800mg) • Evaluated drug kinetics (blood, urine, feces), tolerability and acute safety • Peak blood levels were an area of focus; IV amikacin is known to generate high peak levels with potential kidney - and neuro - toxic effects (hearing loss), therefore safety limits exist for IV amikacin (<10 µg/ml before redosing, peak levels not to exceed 35 µg/ml) - Single - dose MAT2501 amikacin peak levels did not exceed 0.1 µg/ml, leaving more than 100x safety margin; our thesis: this is supportive of meeting this parameter under multi - dosing - Other kinetic data indicating significant absorption and distribution: e.g. single - dose peak levels exceeding 3 µg/ml in urine (30 - 40x blood level), >0.2 µg/ml on Day 2 (>2x peak plasma) • No serious adverse events (AE) reported - Most AEs were of mild and gastro - intestinal nature - These AEs were similar to those seen with MAT2203; not believed to be of antibiotic nature • Tolerability data appear to support 400mg BID dosing; to be confirmed in multiple - ascending dose Phase 1 study (evaluate: kinetics, safety, tolerability) 21
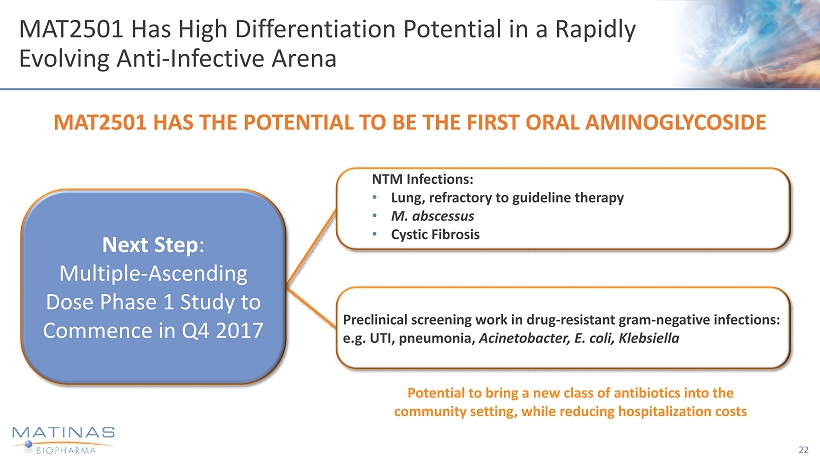
MAT2501 Has High Differentiation Potential in a Rapidly Evolving Anti - Infective Arena 22 MAT2501 HAS THE POTENTIAL TO BE THE FIRST ORAL AMINOGLYCOSIDE Preclinical screening work in drug - resistant gram - negative infections: e.g. UTI, pneumonia, Acinetobacter, E. coli, Klebsiella NTM Infections: • Lung, refractory to guideline therapy • M. abscessus • Cystic Fibrosis Next Step : Multiple - Ascending Dose Phase 1 Study to Commence in Q4 2017 Potential to bring a new class of antibiotics into the community setting, while reducing hospitalization costs
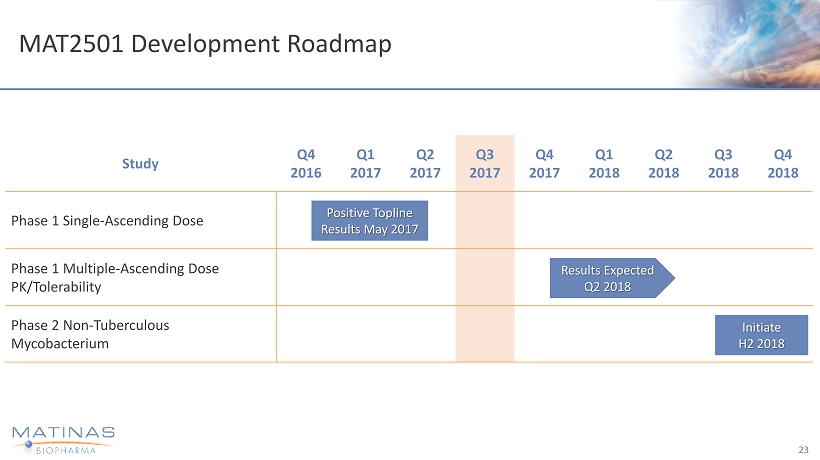
MAT2501 Development Roadmap 23 Study Q4 2016 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 Q3 2018 Q4 2018 Phase 1 Single - Ascending Dose Phase 1 Multiple - Ascending Dose PK/Tolerability Phase 2 Non - Tuberculous Mycobacterium Positive Topline Results May 2017 Results Expected Q2 2018 Initiate H2 2018

Management Team and Board of Directors 24 Roelof Rongen Co - Founder, Chief Executive Officer, Director Jerome D. Jabbour, JD Co - Founder, President Raphael J. Mannino, PhD Chief Scientific Officer Dominick DiPaolo Senior Vice President of Quality and Regulatory Compliance Douglas F. Kling SVP, Clinical Development and Project Management Abdel Fawzy, PhD Co - Founder, EVP, Pharmaceutical & Supply Chain Development Gary Gaglione, CPA VP, Finance, Acting - CFO Management Board of Directors Strong Development and Commercialization Track Record Herbert Conrad Chairman of the Board Roelof Rongen Co - Founder, Chief Executive Officer, Director Jam es S. Scibetta Director Eric J. Ende, MBA, MD Director Stefano Ferrari Director Adam Stern Director Murami Pharma

Prominent Clinical Advisors 25 J. Carl Craft, MD - Chair • Former Chief Scientific Officer for Medicines for Malaria Venture (MMV) • Former Venture Head at Abbott Laboratories Anti - Infective Development Group Prof. Oliver Cornely , MD, FACP, FIDSA • Head of Translational Platform, Principal Investigator, Clinical Trials Center Cologne • President of the European Confederation of Medical Mycology Dimitrios P. Kontoyiannis, M.D., M.S., Sc.D., PhD (Hon),FACP, FIDSA, FECMM • The Texas 4000 Distinguished Endowed Professor For Cancer Research, Department of Infectious Diseases, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX • Frances King Black Endowed Professor, Department of Infectious Diseases, Division of Internal Medicine, The University of Tex as MD Anderson Cancer Center, Houston, TX • Deputy Head Research, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX Peter G. Pappas, MD, FACP • Professor of Medicine in the Division of Infectious Diseases and Tinsley Harrison Clinical Scholar at the University of Alabama in Birmingham • Principal Investigator for the Mycoses Study Group David S. Perlin, PhD • Internationally renowned expert in infectious disease, with primary expertise in fungal infections and mechanisms of antifungal drug resistance • Executive Director of the Public Health Research Institute (PHRI) • Professor of Microbiology, Biochemistry and Molecular Genetics at New Jersey Medical School Public Health Research Institute
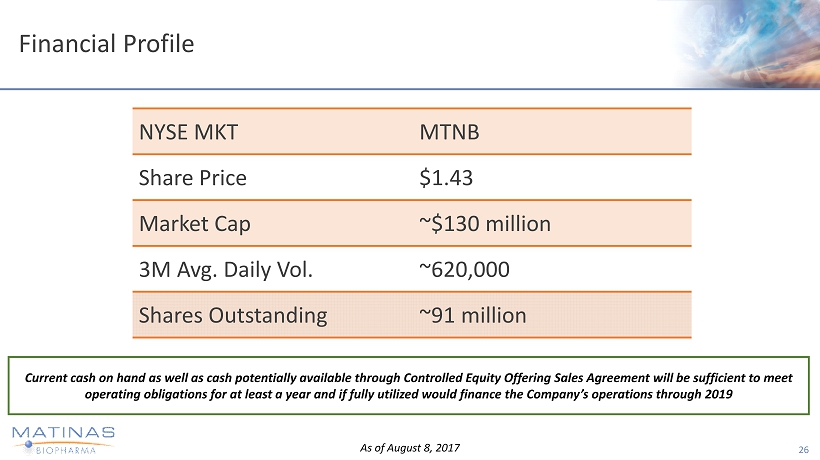
Financial Profile 26 NYSE MKT MTNB Share Price $1.43 Market Cap ~$130 million 3 M Avg. Daily Vol. ~620,000 Shares Outstanding ~91 million As of August 8, 2017 Current cash on hand as well as cash potentially available through Controlled Equity Offering Sales Agreement will be suffici ent to meet operating obligations for at least a year and if fully utilized would finance the Company’s operations through 2019

A Compelling Investment Opportunity x Data generated to date across all studies validates the delivery mechanism of action of drug delivery platform and warrants advancing MAT2203 into a Phase 3 registration study x Company engaging with FDA in Q4 2017 with the goal of moving MAT2203 into Phase 3 as quickly as possible x MAT2203 is poised as potentially game - changing product to become the gold standard and drug of choice to prevent and treat invasive fungal infections. x MAT2501 is potentially first ever oral aminoglycoside with potential to be a solution for a variety of chronic and acute bacterial infections, including gram - negative bacterial infections x The next six, 12 and 18 months will be full of value - creating milestones that we believe will have the potential to drive significant value in Matinas 27

www.matinasbiopharma.com NYSE MKT: MTNB Corporate Presentation August 2017