UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
For
the fiscal year ended
OR
For the transition period from to
Commission
File Number:
(Exact name of registrant as specified in its charter)
| No.
| ||
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered | ||
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes
☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes
☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| ☒ | Smaller reporting company |
Emerging
growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting fi rm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
The
aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which
the common equity was sold on June 30, 2023 was approximately $
As of March 18, 2024, there were shares of the registrant’s common stock, $0.0001 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
MATINAS BIOPHARMA HOLDINGS, INC.
Annual Report on Form 10-K
Fiscal Year Ended December 31, 2023
Table of Contents
| i |
PART I
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report on Form 10-K contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
| ● | our ability to raise additional capital to fund our operations and to develop our product candidates; |
| ● | our anticipated timing for preclinical development, regulatory submissions, commencement and completion of clinical trials and product approvals; |
| ● | our history of operating losses in each year since inception and the expectation that we will continue to incur operating losses for the foreseeable future; |
| ● | our dependence on product candidates which are still in an early development stage; |
| ● | our reliance on our proprietary lipid nanocrystal (LNC) platform delivery technology, and certain related patents which are exclusively licensed to us by Rutgers University (Rutgers); |
| ● | our ability to manufacture GMP batches of our product candidates which are required for preclinical and clinical trials and, subsequently, if regulatory approval is obtained for any of our products, our ability to manufacture commercial quantities; |
| ● | our ability to complete required clinical trials for our lead product candidate and other product candidates and obtain approval from the FDA or other regulatory agents in different jurisdictions; |
| ● | our dependence on third parties, including third parties to manufacture our intermediates and final product formulations and third-party contract research organizations to conduct our clinical trials; |
| ● | our ability to maintain or protect the validity of our patents and other intellectual property; |
| ● | our ability to retain and recruit key personnel; |
| ● | our ability to internally develop new inventions and intellectual property; |
| ● | interpretations of current laws and the passages of future laws; |
| ● | our lack of a sales and marketing organization and our ability to commercialize products, if we obtain regulatory approval, whether alone or through potential future collaborators; |
| ● | our ability to successfully commercialize, and our expectations regarding future therapeutic and commercial potential with respect to, our product candidates; |
| 1 |
| ● | the accuracy of our estimates regarding expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; |
| ● | developments and projections relating to our competitors or our industry; and |
| ● | our operations, business and financial results could be adversely impacted by global instability caused by armed conflicts, pandemics and geo-political uncertainty. |
These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this Annual Report on Form 10-K and are subject to risks and uncertainties. We discuss many of these risks in greater detail under “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this Annual Report on Form 10-K and the documents that we reference and have filed as exhibits to the Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this Annual Report on Form 10-K by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
| Item 1. | Business |
Company Overview
We are a clinical-stage biopharmaceutical company focused on delivering groundbreaking therapies using our lipid nanocrystal (LNC) platform delivery technology (LNC Platform). We are seeking to develop an internal pipeline of products utilizing the LNC Platform to successfully encapsulate small molecules and small oligonucleotides and facilitate targeted and extrahepatic delivery to desired cells and tissues without toxicity. In addition, we believe the LNC Platform can also support building an external pipeline of products for leading pharmaceutical companies seeking to develop novel formulations that capitalize on the unique characteristics of the LNC Platform to facilitate, enhance and optimize the delivery of complex molecules, including small molecules, antisense oligonucleotides (ASOs) and silencing or short interfering RNAs (siRNAs).
Our current lead product candidate is MAT2203 (oral amphotericin B), a highly potent antifungal drug which, by virtue of LNC delivery, has been made oral, safe, and well-tolerated for prolonged administration in patients with life-threatening invasive fungal infections. Following the successful EnACT Phase 2 trial in the treatment of cryptococcal meningitis, MAT2203 is now positioned for a single, Phase 3 registration trial (the ORALTO trial) in support of a New Drug Application (NDA) for the treatment of invasive aspergillosis in patients with limited treatment options.
Additional internal discovery programs are currently directed at:
| 1. | the safe and effective delivery of chemotherapeutic agents for oncology applications; and, | |
| 2. | the formulation and delivery of small oligonucleotides, such as antisense oligonucleotides (ASOs) and silencing or short interfering RNAs (siRNAs), with a primary therapeutic focus on inflammation. |
We are dedicated to maximizing the value associated with our unique LNC Platform. This proprietary platform technology, which partially relies upon an exclusive worldwide license from Rutgers University to certain intellectual property, nano-encapsulates chemical and biological payloads in a manner that facilitates safe, efficient, and targeted intracellular delivery for a wide variety of molecules. LNCs have been used to successfully deliver a variety of therapeutic compounds in vivo, including small molecules, peptides, proteins, and small oligonucleotides. Importantly, LNCs are well differentiated from other lipid nanoparticle delivery technologies.
| 2 |
Our LNCs are primarily comprised of phospholipids, like phosphatidylserine (PS), calcium (which is required to keep LNCs intact) and a designated therapeutic cargo, which is trapped inside or between the bilayers of the LNC, resulting in a highly stable, crystalline structure which protects the cargo and allows for oral administration surviving gastrointestinal conditions. Outside of cells, such as in gastrointestinal fluid and in blood, normal extracellular levels of calcium maintain this crystalline structure, whereas in tissues LNCs are avidly taken up by professional phagocytes, as well as infected, inflamed, and malignant cells. In the much lower calcium environment of the cytosol, LNCs lose their crystalline structure and release their cargo intracellularly.
LNCs have a novel targeting profile utilizing PS to facilitate both membrane fusion and PS-receptor mediated endocytosis. Depending on the therapeutic target being approached, the cargo may either have a direct impact inside that particular cell, or, alternatively blood cells such as neutrophils can act as a vehicle to indirectly deliver cargo to target tissues, as in the case of infection or inflammation. LNCs also have a neutral immunogenic profile enabling repeated administration, unlike lipid nanoparticle (LNP) delivery, which because of inherent cytotoxicity may also not be amenable to repeat administration. Uniquely, the highly stable structure of LNCs permits oral administration (not possible with LNPs) since the cargo is protected within the LNC structure from the harsh environment of the gastrointestinal tract.
Preclinical and clinical studies have consistently demonstrated that orally administered LNCs can successfully deliver therapeutic cargos in vivo, to sites of infection, to areas of inflammation, and to tumors. All these target tissues have cells with PS exposed on their surface (which creates opportunities for direct fusion with the cell membrane), as well as specific PS-recognizing receptors on tissue-resident professional phagocytes (which facilitates additional cellular uptake via endocytosis). The ability to target diseased tissues – and potentially avoid off-target effects - the capability to enter cells via both fusion and endocytic mechanisms, and the ability of LNCs to encapsulate – and deliver - a broad range of therapeutic agents provides a solid foundation from which to create a broad pipeline of internal product candidates and external partnerships. While the delivery of therapeutic nucleic acids has advanced considerably in recent years, to date there has been little progress in orally delivered oligonucleotide therapeutics beyond conjugated oligos specifically targeting the liver. Similarly, there are also significant potential opportunities for both small molecules (with potential for both enhanced safety and increased efficacy) and small oligonucleotides in the oncology area.
MAT2203
Our lead drug candidate based on the LNC Platform is MAT2203, an oral formulation of amphotericin B, a well-known and highly effective antifungal drug. Amphotericin B is currently only available in IV formulations which are associated with significant renal toxicity and have labeled restrictions on their use for up to 2 weeks in the United States and only 1 week in most parts of the world due to toxicities, the most prevalent of which is severe nephrotoxicity. Despite these limitations, amphotericin B is currently used and approved to treat a variety of invasive, and potentially deadly, fungal infections due to its potency. MAT2203, which is formulated using our LNC Platform, has the potential to preserve or even increase the efficacy of amphotericin B, while eliminating the risk of nephrotoxicity and providing more convenient and cost-effective oral administration. MAT2203’s product profile has allowed physicians and patients to use MAT2203 for longer periods of time and more broadly than amphotericin B has ever have been used previously and in an outpatient setting.
MAT2203 has been developed to date with the assistance and financial support of the National Institutes of Allergy and Infectious Disease (NIAID) of the National Institutes of Health (NIH). MAT2203 has been designated as a Qualified Infectious Disease Product (QIDP) with Fast Track Status for the treatment of invasive candidiasis, the treatment of aspergillosis, the prevention of IFIs in patients who are on immunosuppressive therapy, and, most recently with an Orphan Designation for the treatment of cryptococcosis. We plan to pursue additional orphan designations for the treatment of aspergillosis, the treatment of invasive candidiasis and the treatment of certain endemic mycoses. Upon approval, MAT2203 could be eligible for up to 12 years of regulatory or marketing exclusivity in the United States.
The initial indication for MAT2203 is early step-down therapy from IV amphotericin B for the treatment of invasive aspergillosis in patient with limited treatment options. Invasive aspergillosis is a serious and life-threatening invasive fungal infection that occurs primarily in severely immunocompromised patients with hematological malignancies and in transplant recipients. This initial step-down indication is a gateway indication, as we plan to expand the utilization of MAT2203 into the treatment of other invasive fungal infections (IFIs) and potentially even for prophylaxis against IFIs in immunocompromised patients, such as transplant patients.
| 3 |
The EnACT (Encochleated Oral Amphotericin for Cryptococcal Meningitis Trial) Phase 2 study was a Phase 2 prospective, randomized, open-label, sequential cohort study, financially supported by the NIH NINDS, evaluating the safety, tolerability, and efficacy of MAT2203 in 100 HIV-positive persons with cryptococcal meningitis. The EnACT trial included a total of four cohorts of patients, with the first two cohorts testing MAT2203 as early step-down therapy following initial treatment with IV amphotericin B during the 14-day induction period, and the second two cohorts testing MAT2203 as potentially all oral therapy. The induction period for all patients in each cohort (active or control) is 14 days, followed by an additional four weeks of treatment (active or control) during a consolidation/maintenance period. Cohorts 1 and 3 were safety lead-ins to Cohorts 2 and 4, respectively, which were the key efficacy cohorts for EnACT.
The primary endpoint in EnACT was Early Fungicidal Activity (EFA), a measurement of cerebrospinal fluid fungal clearance. EFA is a well-validated quantitative measure of the efficacy of antifungal agents and is a key surrogate marker for survival. EFAs of less than 0.20 log10 Cryptococcus colony forming units (CFUs) per mL CSF per day are associated with significantly higher mortality and worse clinical outcomes1. EFA measured above this threshold is clinically meaningful and represents robust fungal clearance. In the second cohort of EnACT, the mean EFA achieved with patients treated with MAT2203 was 0.38 log10 CFU/mL/day, with 95% confidence intervals (0.30 to 0.46) significantly higher than the prespecified primary endpoint threshold of >0.20. All patients treated with MAT2203 who completed the induction phase achieved sterile CSF cultures during treatment (either during induction or early consolidation phases). There was no evidence of breakthrough or relapsed cryptococcal infections observed in any of the patients during treatment with MAT2203 through 10 weeks. In Cohort 2, overall survival was 90% after 18 weeks in 40 patients randomized to receive MAT2203.
Interim data from Cohort 4 of the Phase 2 EnACT study of MAT2203 (oral amphotericin B) for the treatment of cryptococcal meningitis (CM) were presented at IDWeek in October 2022. As part of IDWeek, the EnACT abstract was the recipient of the Outstanding Abstract and IDSA Awardee by the Infectious Diseases Society of America. In the EnACT trial, MAT2203 exceeded the primary endpoint threshold for early fungicidal activity (EFA) of 0.20 log10 CFU/mL/day, with a mean EFA achieved of 0.30 log10 CFU/mL/day with 95% confidence intervals from 0.22 – 0.38.
Cohort 4 also yielded key secondary endpoints, including overall survival and safety. For 40 patients receiving MAT2203 treatment, overall survival remained at 90% through 18 weeks, while the survival rate at Week 2 was 95% (the primary endpoint for the upcoming Phase 3 registration trial in cryptococcal meningitis). Importantly, the incidence of adverse events relating to kidney function and anemia were significantly lower for MAT2203 compared to the conventional IV amphotericin B standard of care treatment across the entirety of the EnACT trial, with no evidence of kidney toxicity even with up to 6 weeks of oral MAT2203 treatment.
In February 2024, we announced agreement with the United States Food and Drug Administration (FDA) on the design of a single Phase 3 registration trial of MAT2203 in patients with invasive aspergillosis who have limited treatment options, including consensus on all critical elements of the registrational path for MAT2203 (the ORALTO trial).
ORALTO is a Phase 3, randomized, multicenter, open-label, adjudicator-blinded study to evaluate the efficacy and safety of MAT2203 as an oral step-down treatment following treatment with AmBisome® (liposomal IV-amphotericin B) compared with the standard of care in patients with invasive aspergillosis who have limited treatment options. The primary efficacy endpoint is all-cause mortality at study day 42.
Key secondary objectives include:
1*Clin Infect Dis. 2020;71(5):e45-49
| 4 |
| (a) | demonstration of superiority of oral-step down treatment with MAT2203 compared with AmBisome for treatment-related toxicities leading to changes in treatment (i.e., dose adjustment/discontinuations or changes to treatment regimens); | |
| (b) | long-term survival benefit of MAT2203 using all-cause mortality at study day 84; | |
| (c) | evaluation of the impact of MAT2203 on healthcare resource utilization and quality of life impact. |
Enrollment is expected to include approximately 216 adults with recently diagnosed probable or proven invasive aspergillosis who are being treated with AmBisome due to their inability to receive an IV mold-active azole and with limited alternative treatment options. Following up to two days of initial treatment with AmBisome, eligible study participants will be entered into the study and randomized in a 2:1 ratio to receive either oral MAT2203 or continued AmBisome treatment followed by standard of care.
All study participants will receive up to 12 weeks of treatment starting from the first day of treatment with AmBisome. It is anticipated that all study participants will be hospitalized during the initial AmBisome treatment period. After step-down to oral MAT2203, study participants may be discharged from the hospital to continue treatment on an outpatient basis, as clinically appropriate.
An independent Data Review Committee, who will be blinded to treatment, will adjudicate primary and secondary endpoints, including clinical, radiological, and mycological responses. Once approximately 75% of participants are enrolled, an independent Data Safety Monitoring Board will review the overall pooled all-cause mortality rate in a blinded fashion to ensure that the sample size assumptions are reasonable and that the study is adequately powered. Should the pooled event differ substantially from expected levels, a sample size adjustment can be made to the trial.
ORALTO will be conducted at approximately 65 investigator sites in the U.S., Europe, South America, Middle East, and Asia Pacific. Enrollment is expected to commence in the second half of 2024 and is expected to require approximately 24 months.
We are actively engaged in an ongoing partnership process for MAT2203, seeking one or more development and/or commercialization partners. We will require either (i) the consummation of a partnership transaction, or (ii) raising additional capital, prior to commencing the ORALTO trial.
In addition to conducting the EnACT trial, a MAT2203 Compassionate/Expanded Use Access Program was established to provide MAT2203 on a compassionate use basis. Enrollment into the Program requires that patient applicants meet certain criteria for eligibility, including:
| ● | the patient has no other treatment options. |
| ● | the invasive fungal infection is serious and/or life-threatening. |
| ● | the patient is expected to benefit from oral MAT2203 treatment and can tolerate oral medication; and |
| ● | the patient has a reasonable life expectancy, and their underlying conditions are under control. |
19 patients to date have been enrolled in the Program at multiple healthcare institutions, including the University of Michigan, Johns Hopkins, Nationwide Children’s Hospital, City of Hope, Vanderbilt University Medical Center, the National Institutes of Health, Children’s Hospital of Philadelphia, Memorial Sloan Kettering Cancer Center, and the University of California, San Diego School of Medicine. The majority of enrolled patients are post-transplant or are undergoing treatment for underlying malignancies. The infections being treated with MAT2203 include a variety of micro-organisms (including Aspergillus, Mucorales species, Candidiasis, Fusarium and suspected Coccidioides) occurring at multiple sites of infection, including brain, bladder/colon, bone, lung, sinus, and skin. Most patients were receiving AmBisome® prior to enrollment but developed treatment-limiting nephrotoxicity and most also required treatment for either azole-resistant organisms or had clinically failed azole therapy and had no other treatment options.
Of the 19 patients enrolled in the Program, 15 have available follow-up and 4 recently initiated or will soon commence treatment with MAT2203.
| 5 |
| ● | 12 of the 15 patients had either complete clinical resolution or objective improvement in clinical markers of infection (including radiologic and mycologic). | |
| ● | Of the 5 patients who completed their full individually specified course of treatment (ranging from 2 weeks to 1 year, depending on the infection) - all have had complete clinical resolution with no relapses or recurrence of their infection. | |
| ● | 5 additional patients have shown objective improvement in clinical markers and are continuing treatment with MAT2203 as planned. | |
| ● | 5 of the 15 patients were unable to complete their individually specified course of treatment, although 2 saw significant clinical improvement of their fungal infection and are included in the 12 overall successful cases. |
| – | 2 patients transitioned to palliative care shortly after starting therapy with MAT2203 because of unanticipated progression of their malignant disease. | |
| – | 1 patient discontinued therapy after two days due to underlying GI issues (i.e., Crohn’s disease). | |
| – | 1 patient passed away due to progression of their underlying disease approximately 8 weeks into therapy (but previously experienced significant clinical improvement of their fungal infection). | |
| – | 1 patient discontinued MAT2203 treatment following 10 weeks of therapy due to underlying GI issues (long-standing nausea/vomiting), but with improvement in their fungal infection. |
Importantly, all patients who experienced renal toxicity following treatment with AmBisome saw their renal function return to baseline after transitioning to MAT2203 therapy and suffered no further renal side effects over the course of extended treatment with MAT2203.
LNC Platform Work
In addition to advancing MAT2203 into the ORALTO Phase 3 trial, we continue to expand the utilization of the LNC Platform with small molecules and small oligonucleotides outside of infectious disease, targeting inflammation and oncology.
Inflammation
We are investigating a variety of LNC formulations of two small oligonucleotides designed to target inflammatory cytokines IL-17A and TNFα and have conducted a series of in vitro and in vivo studies evaluating the biological activity associated with oral delivery as well as the corresponding associated clinical benefit of IL-17A knockdown in an imiquimod (IMQ) induced murine psoriasis disease model.
These preliminary studies have documented biological activity in the form of cytokine knockdown and have also provided some evidence of associated tangible clinical benefit with improvements in skin lesion appearance (redness, scaling) in this qualitative psoriasis model. These data remain under evaluation and the analyses are focused on (a) clarifying the strength and time course of cytokine inhibition, and (b) evaluating cytokine mRNA levels and specific tissue responses in these models to better understand and interpret these data.
In December 2023, we announced results from a series of in vivo studies demonstrating successful oral delivery of two LNC-formulated small single-strand oligonucleotides that specifically target key inflammatory cytokines TNFα and IL-17A in well-established and validated animal models that mimic acute inflammatory responses seen in human diseases.
| 6 |
Acute Colitis Study (“TNFα”)
A dextran sulfate sodium (“DSS”)-induced murine colitis model was used to evaluate an orally administered LNC-delivered small oligonucleotide that specifically targets TNFα mRNA synthesis. Colon tissue TNFα mRNA levels, as assessed by quantitative real-time PCR analysis, were lower following orally administered active LNCs, resulting in statistically significant reductions of serum TNFα levels by 37% compared with diseased, but untreated animals. Importantly, clinical disease activity scores at key time points in the studies were also significantly improved with an active LNC formulation.
Acute Psoriasis Study (“IL-17A”)
An imiquimod (“IMQ”)-induced murine psoriasis model was used to evaluate an orally administered, LNC-delivered small oligonucleotide designed to inhibit IL-17A mRNA synthesis, which contributes significantly to the progression of psoriatic skin lesions. Similar to the DSS colitis model, skin tissue levels of IL-17A mRNA in the IMQ psoriasis model were lower with orally administered active LNCs compared with IMQ alone. In this model, while IL-17A serum levels were not expected to change, improvement was demonstrated in clinical disease markers of skin redness and scaling, further validating the biological activity of these small oligonucleotides.
Oncology
In November of 2023 we announced positive results from an in vivo animal study of an oral LNC formulation of docetaxel, a well-known chemotherapeutic agent used in the management of multiple metastatic and unresectable tumors. Anti-tumor effects of daily oral LNC-docetaxel were comparable to IV-docetaxel with statistically significant reductions in tumor volume compared with untreated controls at Day 14 (high dose oral LNC -63%; low dose oral LNC -57%; IV docetaxel -68%), and similar reductions in tumor weight at Day 14. No systemic toxicities were noted. Body weight was stable over treatment duration and hematologic parameters were similar to untreated controls.
In March 2024 we announced positive results from an additional in vivo study of LNC-docetaxel in healthy mice. The goal of the study was to determine whether an oral LNC formulation of docetaxel could improve the overall safety profile of conventional IV-administered docetaxel. The study included 24 healthy BALB/c mice divided into three treatment groups: (1) control animals treated with oral saline, (2) IV-docetaxel (30 mg/kg, or 0.6 mg/dose) administered once a week for three weeks, and (3) oral LNC-docetaxel (37.5 mg/kg, or 0.75 mg/dose) administered once daily over three weeks. The primary endpoint of the study was change in body weight over the treatment period which how toxicity is primarily manifested in this model.
Key takeaways included the following:
| ● | Through Day 22, the total amount of docetaxel administered with the oral LNC-docetaxel formulation was more than 8x greater than with IV-docetaxel (the final IV dose was used to measure pharmacokinetics). | |
| ● | All mice treated with IV-docetaxel lost a significant amount of weight (toxicity), with an average peak loss of 20% of their original body weight. | |
| ● | Mice treated with oral LNC-docetaxel maintained their body weight, which was statistically no different than the weight of control mice treated with oral saline. | |
| ● | One mouse in the IV-docetaxel group died prior to the conclusion of the study and was censored from the analysis (last measurement was -32% body weight loss); the curve of the IV-docetaxel group also reflects the expected 7-day recovery following an IV dose of docetaxel. | |
| ● | The daily administered oral LNC-docetaxel dose was 50% higher, and the total amount of drug administered was 3.5 times greater, than the LNC-docetaxel dose administered in a previous study that demonstrated anti-tumor activity comparable to IV-docetaxel in a syngeneic mouse melanoma model. |
Next steps include evaluating the efficacy of the current LNC-docetaxel formulation in other tumor models. Additionally, we plan to evaluate the potential anti-tumor activity of LNC formulations of small oligonucleotides.
| 7 |
Strategy
We are focused on redefining the intracellular delivery of nucleic acids and small molecules through our LNC Platform and its application to overcome current challenges in safely and effectively delivering small molecules, nucleic acids, gene therapies, proteins/peptides, and vaccines.
Key elements of our strategy include:
| ● | Advancing MAT2203 into the ORALTO trial for the treatment of invasive aspergillosis in patients with limited treatment options by securing a development and/or commercial partner. This initial indication is designed to be a gateway indication to establish the pharmacodynamic bridge necessary to expand the use of MAT2203 into other indications to treat deadly invasive fungal infections (e.g., mucormycosis, candidiasis and the endemic mycoses) through limited additional clinical work under a 505(b)(2) pathway, thereby making MAT2203 a pipeline in a product. |
| ● | Expanding the utilization of our LNC Platform with other small molecules and small oligonucleotides into inflammation and oncology in order to develop an internal pipeline of differentiated drug candidates. Oral, extrahepatic and non-toxic intracellular delivery of these molecules would represent a significant advancement. |
| ● | Building an external pipeline of collaborations focused on our LNC Platform with leading pharmaceutical companies to provide delivery solutions for their complex small molecules and small oligonucleotides, including ASOs and siRNAs. |
Our Lipid Nanocrystal (LNC) Platform
Safe, efficient, and targeted intracellular delivery of medicines remains one of the biggest challenges in the pharmaceutical and biotech industry today. An ever-growing understanding of the complex biology within cells has given rise to increasingly sophisticated therapeutic approaches targeting the genetic machinery driving metabolic activity within cells. However, challenges remain, especially for small molecules causing significant toxicity and for therapeutic oligonucleotides in areas including safely and efficiently delivering cargo to the interior of a cell, delivering to targets beyond the liver and in providing opportunities for longer-term oral delivery to increase convenience and resulting in a potentially significant pharmacoeconomic impact.
Strategically, we are seeking to leverage the unique features of the LNC Platform and the biology of phosphatidylserine to extend potential applications for the intracellular delivery of highly potent, but highly toxic small molecules as well as the oral, target delivery of small oligonucleotides.
LNCs – Background
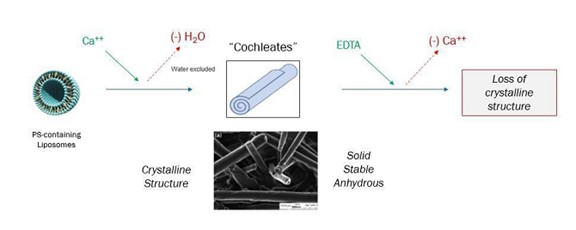
“Cochleate lipid cylinders” were originally described in scientific literature as complex, cylindrical, multi-lamellar structures arising from the addition of Ca++ to sonicated liposomal preparations of phosphatidylserine (PS) in an aqueous solution, with the lamellae folded in a spiral, crystalline configuration that excludes water. When sufficient external calcium is present, these structures remain in a crystalline state, while the addition of etheylenediaminetetraacetic acid (EDTA) to these preparations (removing the calcium) results in the loss of the stable spiral crystalline structure (Figure 1).
| 8 |
Figure 1: Cochleate Formation

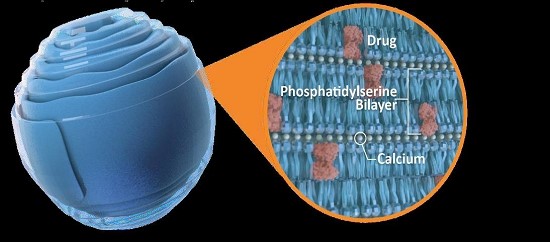
We have developed techniques to embed cargo molecules within cochleates as they are assembled. These new cargo-carrying structures (termed lipid nanocrystals, or LNCs) have been used to successfully deliver several different cargo molecules to cells in vitro and to animals and humans in vivo. Because of their exceptional stability (an anhydrous crystalline structure) and unique composition (PS-containing bilayers), we believe that LNCs are a promising alternative for the intracellular delivery of a variety of small molecules – proteins, peptides – and small oligonucleotides such as ASOs and siRNA. In addition, since the normal physiologic levels of calcium in the gut can maintain their crystalline structure, LNC formulations can also potentially be delivered orally, as the encapsulated cargo is protected from degradation by harsh environmental conditions or enzymes (Figure 2).
Figure 2: LNCs Encapsulate and Protect their Cargo in a Water-free Environment
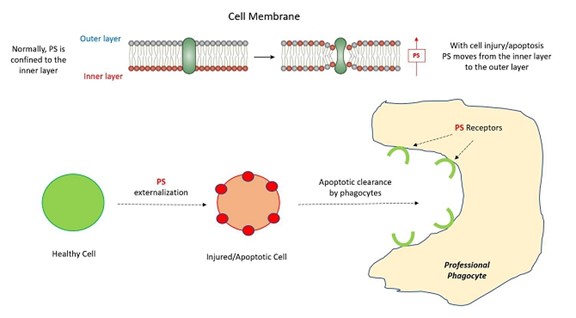
Importance of Phosphatidylserine
Phosphatidylserine (PS) is present in virtually all cells and is an integral part of the cell membrane. PS is normally localized to the inner part of the membrane bilayer by active cellular processes and not normally exposed externally (Figure 3). However, when cells are injured and/or apoptotic, PS moves from the inner layer to the outer layer and is the primary “eat me” signal driving efferocytotic clearance of apoptotic cells by professional phagocytes.
Studies with cargo carrying LNCs have documented both a lack of cargo accumulation in the tissues of normal, healthy animals, and significant cargo delivery to involved tissues in infected animals. Another important aspect of the underlying stability of LNCs is that they do not release their cargo within the blood (due to the presence of stabilizing levels of calcium), and delivery of cargo is confined to involved cells and tissues.
| 9 |
Mechanistically, the cellular entry of LNCs is driven by PS, which is an important participant in both efferocytotic clearance of apoptotic cells and in physiologic cellular fusion processes (Figure 3). As noted, apoptotic cells expressing PS on their surface are recognized and cleared by professional phagocytes, without eliciting the normal inflammatory responses that might otherwise be anticipated with cell death. Professional phagocytes themselves have several very specific PS receptors that facilitate this clearance. Parenthetically this mechanism is exploited by enveloped viruses to facilitate viral uptake by immune cells – “viral apoptotic mimicry” – noting that the “envelope” of enveloped viruses is basically comprised of PS. Thus, phagocytosis of LNCs by professional phagocytes (and some non-professional phagocytes) becomes one mechanism for intracellular delivery of LNCs.
Figure 3: Importance of Phosphatidylserine (PS) in apoptotic phagocytosis
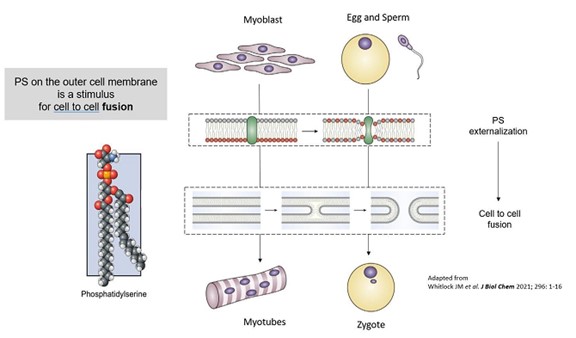
As described above, in addition to its role as an “eat-me” signal for uptake of apoptotic cells by phagocytes, PS also plays a very important role in normal physiologic cellular fusion processes – as a “fuse-me” signal (such as with the formation of myotubules from myoblasts, the formation of osteoclasts from osteoblasts, and the fertilization of an of egg by sperm to form a zygote) or even as a “heal me” signal (as with axonal fusion after injury or repair of injured cell membranes). Consequently, the presence of PS on both the surface of LNCs and on the surface of targeted somatic cells (which themselves may express PS because of injury or inflammation) creates additional opportunities for intracellular delivery via direct cellular fusion (Figure 4).
| 10 |
Figure 4 – Importance of Phosphatidylserine (PS) in Cellular Fusion
LNC Drug Delivery
Because of their underlying stability, LNCs do not release cargo within the blood (due to the presence of stabilizing levels of calcium), and the delivery of cargo is confined to involved cells and tissues. Studies utilizing an LNC formulation of radio-labeled amphotericin showed no accumulation in normal healthy tissues, in contrast to multiple studies showing clinically meaningful tissue levels of amphotericin when amphotericin-carrying LNCs were administered in the setting of systemic fungal infections.
After oral administration, LNCs are transported across the cells lining the gastrointestinal tract (via transcytosis). Because of their size, LNCs do not enter the portal circulation and thereby avoid first pass hepatic metabolism. Instead, they are transported through the gut lymphatics to the thoracic duct and enter the circulatory system via the superior vena cava. Once they have entered the circulation, LNCs are transported by professional phagocytes (as well as via other non-cellular mechanisms) to sites of injury or infection, where they are avidly taken up by infected/injured cells that have PS on the outer layer of their cell membranes. Finally, when exposed to the very low calcium environment in the interior of a cell, the forces responsible for maintaining the otherwise stable LNC structure are no longer as strong, and the LNCs release their cargo.
Differentiation from LNPs
Conventional LNPs in blood bind ApoE to their surface and are taken up into cells by clathrin-mediated endocytosis via the ApoE-recognizing LDL receptor. From within early endosomes, LNPs then act to disrupt the endosomal membrane and gain entry to the cytosol. Endosomal escape of LNPs is a very inefficient process, with endosomal escape rates of generally < 5%. Administration of LNPs can also be associated with injection site reactions and other toxicities arising from the destruction of the endosomal membrane, which, ultimately, can limit their chronic use. Additionally, LNPs cannot be delivered orally. LNC delivery can overcome many of the limitations of LNPs with oral administration, extra-hepatic targeting, multiple potential cellular entry mechanisms, a breadth of payload capabilities, the ability to target both immune and somatic cells, reduced toxicity/improved safety, and opportunities for safe, chronic long-term administration. Each of these have been validated with the clinical success of MAT2203 in the treatment of deadly invasive fungal infections.
| 11 |
Therapeutic Targets
Infection
The therapeutic applications of our proprietary delivery technology have focused initially on the delivery of potent, highly effective anti-infective agents that have treatment-limiting potential toxicities, including irreversible toxic effects on kidney and hearing function. In MAT2203, we have developed a less toxic and orally bioavailable formulation of the potent (but otherwise highly toxic) fungicidal drug amphotericin B that can be safely used for longer periods of time than currently possible with conventional amphotericin in the treatment of deadly fungal infections. This, in turn, has created potential opportunities to use amphotericin B in ways that were not previously possible with conventional formulations, and create opportunities for amphotericin treatment in settings where conventional amphotericin has proven to be too toxic, and open the possibilities for much longer-term treatment which should result in overall improved outcomes for patients with a significant pharmacoeconomic impact.
Inflammation
With the observation that LNCs are avidly taken up by innate immune cells (monocytes, neutrophils, and dendritic cells) we believe that LNCs could become very useful in treating inflammatory diseases. We have gained access to a number of small oligonucleotides that are capable of very selectively knocking down individual cytokines – specifically IL-17A and TNFɑ. We have successfully advanced the program with these small oligos, first with successful, efficiently encapsulated LNC formulations, and next with in vitro validation of the efficacy of the LNC formulations. More recently, we have progressed two of these LNC-formulated small oligos into in vivo studies in animal disease models, with successful demonstration of both measurable biological activity and potential therapeutic efficacy in two different models. In a murine imiquimod-induced psoriasis model we observed reductions of tissue IL-17A mRNA, accompanied by improvement in skin redness and scaling. Similarly, in a murine DSS model of inflammatory bowel disease, daily oral administration of an oral LNC formulation of the TNFɑ targeted oligonucleotide resulted in reductions of colon TNFɑ mRNA, significant reductions in serum TNFɑ levels, and significant improvements in disease activity scores.
Cancer
Building on the success of MAT2203 in creating a safe, effective formulation of amphotericin that can be administered orally for much longer periods of time for the treatment of deadly fungal infections, and having seen how avidly tumor cells can take up LNCs in vitro, we have extended the application of LNC technology to oncology applications. Some tumors cell lines are also known to have relatively high levels of surface PS expression, which provides potential directionality for those tumors which may respond positively to treatment with LNC-mediated chemotherapies. Building upon our experience with MAT2203, there are two specific areas where we believe that LNCs could provide major advances:
| 1) | potential for reduced toxicity because of tissue-targeted delivery and low blood levels | |
| 2) | potential for enhanced efficacy through more efficient delivery |
We began initial formulation work with a number of chemotherapeutic agents, and ultimately chose to move forward with docetaxel. We have shown in vitro therapeutic efficacy that proved even better than conventional docetaxel, and moved rapidly to in vivo studies and into a syngeneic mouse melanoma model (with known high surface PS expression). In this model, our oral LNC formulation of docetaxel proved to be equally effective as conventional intravenous docetaxel. Moreover, despite daily oral administration of the LNC formulation, there was no adverse impact on body weight, and no indications of any hematologic toxicity. Through this work, we have demonstrated that an oral LNC formulation of docetaxel, administered daily, can deliver levels of drug to tumors provide a comparable therapeutic response to that of conventional IV docetaxel, without any indications of toxicity. Additional in vivo work in healthy mice has shown that even higher doses of LNC docetaxel (50% greater) were equally free from toxicity, while similar increases in the IV docetaxel dose were accompanied by indications of toxicity, with a mean weight loss of approximately 20%.
| 12 |
Our LNC Clinical Stage Asset: MAT2203
Our lead product candidate, MAT2203, is an orally administered LNC formulation of a broad spectrum anti-fungal drug called amphotericin B. Traditionally, amphotericin B is an IV-administered drug used as a last resort for treatment of systemic fungal infections resistant to triazoles and echinocandins, including resistant candidiasis, cryptococcal meningoencephalitis, and aspergillosis. To date, there have been little to no reported clinically observed drug-resistance to amphotericin B, further bolstering the use of this compound as the most likely last resort treatment for fungal infections in the foreseeable future. However, the use of amphotericin B is relatively limited because it is currently only available as an IV-administered product and has documented history of severe toxicity (most notably nephrotoxicity). By utilizing our LNC Platform to nano-encapsulate amphotericin B, we have created an opportunity for the drug to be administered orally with targeted delivery to infected cells and tissues, which we believe may have fewer side effects than the currently available IV-formulations of amphotericin B, and potentially result in greater efficacy due to the ability to administer amphotericin B safely for longer periods of time.
Our LNC delivery of amphotericin B changes the bio-distribution, resulting in a higher level of amphotericin B at the site of infection and a lower level of free circulating drug. By reducing the amount of circulating drug, our LNC Platform may reduce overall toxicity. Importantly, drug concentrations will be high only in target tissues due to the migratory nature of drug-carrying phagocytes to inflammatory regions. Based upon data generated to date, we believe MAT2203 has the potential to offer improved safety and reduced toxicity, and, as a result, we believe MAT2203 will be able to offer a categorically different and improved formulation that delivers orally administered amphotericin B, directly to the target cell at the site of infection.
The data from animal toxicity and human studies for MAT2203 indicate a substantial advantage over other amphotericin B formulations in observed toxicities and side effects, which we believe is driven by two primary factors:
| ● | The LNC is a solid crystal that does not significantly “leak” its drug content while circulating. The crystal releases its medication pay-load only when inside the target cells, and thus appears that the use of MAT2203 does not result in off-organ toxicities normally seen in the kidneys when using current formulations of amphotericin B. |
| ● | Because of this targeted delivery, we have been able to increase the therapeutic window on a mg/kg basis as compared to IV amphotericin B formulations. We have observed equivalent efficacy at lower doses as well as been able to use oral doses of up to 10x the highest tolerable IV dose in animal model studies. |
The profile of MAT2203, which facilitates the oral, targeted, and non-toxic delivery of amphotericin B has the potential to fundamentally change the treatment paradigm for IFIs, which are a rapidly growing global threat.
The Increasing Problem of Fungal Pathogens
Fungal pathogens and infections are an increasing global public health concern. IFIs are increasing globally due to advancements in the medical management of critically ill and immunocompromised patients. The emergence of drug-resistant IFIs has resulted in prolonged hospitalizations and the increased use of expensive and often highly toxic second-line antifungal agents.
Fungal pathogens cause a wide variety of infections but there are only four currently available classes of systemic antifungal treatments (polyene, azole, echinocandin, pyrimidine), and only two classes (azole, pyrimidine) are available in oral formulations. Use of these antifungal agents typically require a considerable degree of expertise to manage potential toxicities and complex drug-drug interactions in these vulnerable patients and effective polyene and echinocandins require prolonged hospitalization for intravenous administration. Therefore, there is a critical unmet need for more effective, well-tolerated, and safe oral antifungal agents to treat patients with serious, life-threatening, and often drug resistant IFIs.
The World Health Organization (WHO) recently recognized IFIs to be a global public health concern. In late 2022, the WHO released their Fungal Priority Pathogen List which designates Aspergillus fumigates, Candida auris, and Candida albicans to be in the Critical Priority group (i.e., highest perceived public health threat), Mucorales, Candida tropicalis, and Candida parapsilosis in the High Priority group, and Coccidioides species in the Medium Priority group. Aspergillus, Candida, Coccidioides, and Cryptococcus species are also qualified pathogens that pose a serious and life-threatening risk and are on the qualified designation list according to 317.2 – CFR – Code of Federal Regulations Title 21 – FDA.
| 13 |
We believe that MAT2203 has the potential to become a best-in-class therapy for the treatment of IA and for other IFIs more broadly by offering the following key potential benefits:
| ● | Potential to treat resistant pathogens. We believe that MAT2203 has the potential to prevent and treat fungal infections caused by drug-resistant fungi, including those resistant to existing azoles and echinocandins, due to amphotericin B’s fungicidal (i.e., killing the fungi) nature and potency against resistant strains and the potential for our LNC Platform to provide higher drug exposure directly at the site of infection early during therapy. |
| ● | Enabling an all-oral therapy. The ability to step-down to an oral amphotericin B product early during treatment of an invasive fungal infection will offer significant benefit to patients for whom there are only limited oral treatment options that carry significant issues of safety, tolerability, and high propensity of resistance, as seen with the azole class of antifungals. |
| ● | Shorter and less costly hospital stays and lower outpatient costs. By providing physicians and patients with access to an orally available, broad spectrum fungicidal agent in MAT2203, we believe that there is significant potential to reduce hospital costs, which account for over 70% of the overall treatment cost of IFIs. |
MAT2203 Development History and Plan
Preclinical Data
MAT2203 has demonstrated antifungal activity when administered orally in several animal models for Cryptococcus, Candida, and Aspergillus infection [Zarif et al, 2000; Perlin, 2004; Lu et al, 2019]. The efficacy in these animal models has been shown to demonstrate comparable or superior antifungal activity compared to IV amphotericin B but with reduced toxicity.
The in vivo efficacy of MAT2203 has been shown in multiple mouse models infected with Cryptococcus neoformans; these studies were conducted by Dr. Peter Williamson at NIH [Lu et al, 2019]. Multiple studies demonstrated the potential for MAT2203 to be administered in combination with 5FC to provide an effective oral formulation for treatment of cryptococcal meningitis. Using a 3-day delayed model of murine cryptococcal meningoencephalitis and a large inoculum of a highly virulent strain of serotype A C. neoformans, MAT2203, administered in combination with 5FC, was found to have efficacy equivalent to administered amphotericin B deoxycholate with 5FC and superior to oral fluconazole without any observed toxicity. The transport of fluorescent MAT2203 particles to the brain as well as significant brain levels of amphotericin drug was demonstrated in treated mice, and immunological profiles were similar to those of mice treated with conventional amphotericin B. These studies suggest the potential for an efficacious oral formulation of a known fungicidal drug against intrathecal cryptococcal disease. MAT2203 therefore provides a promising therapeutic option for CM.
Additional preclinical studies of MAT2203 have been conducted to investigate the treatment of other IFIs. Oral MAT2203 was demonstrated to be effective in multiple nonclinical studies. In vitro studies have demonstrated that the MAT2203 LNC formulation did not have an impact on the antifungal activity of amphotericin B. Oral MAT2203 was demonstrated to be effective in several mouse models of systemic fungal infection with Candida, Aspergillus, and Mucor. In the Candida infection models in both immunocompromised and immunocompetent mice, the efficacy of oral MAT2203 was comparable to intraperitoneal (IP) amphotericin B in terms of survival and reduction of tissue burden in target organs of lung, liver, and kidneys of infected animals. In the Aspergillus mouse models of infection, the efficacy of oral MAT2203 was comparable to intraperitoneal amphotericin B in terms of survival, with dose-dependent reduction of fungal tissue burden. Oral MAT2203 also demonstrated in vivo efficacy in treating R. delemar or M. circinelloides pulmonary infections in immunosuppressed mice and led to a reduction in fungal spores in lung and brain comparable to results achieved with the liposomal intravenous amphotericin B.
| 14 |
EnACT Phase 2 Trial
Based a comprehensive preclinical data package, the NIH financially supported a grant application from the University of Minnesota to conduct the EnACT Phase 2a/b trial in Uganda. This study was initiated in October 2019 and explored the use of MAT2203 for both induction and maintenance therapy in the treatment of cryptococcal meningitis, which is one of the most frequent and opportunistic infections in HIV patients. Given the high morbidity and mortality associated with CM in HIV patients, the clinical unmet need is very high with the global burden estimated at 1 million cases annually.
EnACT consisted of two parts. Part 1 of EnACT was conducted in HIV-positive patients with a history of cryptococcosis, evaluated ascending oral doses of MAT2203, and identified a safe maximum tolerated dose for Part 2 of the trial.
Part 2 of EnACT was a prospective, randomized, open-label sequential cohort clinical trial to investigate the safety, tolerability, and efficacy of oral MAT2203 in 100 HIV-positive persons with cryptococcal meningitis compared to SOC. The EnACT trial included a total of four cohorts of patients, with the first two cohorts testing MAT2203 as early step-down therapy following initial treatment with IV amphotericin B during the 14-day induction period, and the second two cohorts testing MAT2203 as potentially all oral therapy. The induction period for all patients in each cohort (active or control) is 14 days, followed by an additional four weeks of treatment (active or control) during a consolidation/maintenance period. Cohorts 1 and 3 were safety lead-ins to Cohorts 2 and 4, respectively, which were the key efficacy cohorts for EnACT.
Cohort 2 of EnACT was designed to assess the potential to treat CM infections with oral MAT2203 as a step-down treatment during the induction phase of treatment immediately following only two days of IV amphotericin treatment, with continued treatment with MAT2203 for up to six weeks during early maintenance treatment. The primary efficacy endpoint for Part 2 of EnACT was early fungicidal activity (EFA) defined as rate of clearance of Cryptococcus from the cerebrospinal fluid (CSF) (log10 colony forming units [CFU]/mL/day) as measured by serial quantitative fungal cultures over the first two weeks of treatment.
In Cohort 2 of EnACT, we reported the following results:
Potent Early Fungicidal Activity (EFA), Cerebrospinal Fluid (CSF) Sterilization, with No Evidence of Breakthrough Infections During Treatment with MAT2203
| ● | The primary endpoint in EnACT was EFA, a measurement of cerebrospinal fluid fungal clearance. EFA is a well-validated quantitative measure of the efficacy of antifungal agents and is a key surrogate marker for survival. EFAs of less than 0.20 log10 Cryptococcus colony forming units (CFUs) per mL CSF per day are associated with significantly higher mortality and worse clinical outcomes2. EFA measured above this threshold is clinically meaningful and represents robust fungal clearance. In the second cohort of EnACT, the mean EFA achieved with patients treated with MAT2203 was 0.38 log10 CFU/mL/day, with 95% confidence intervals (0.30 to 0.46) significantly higher than the prespecified primary endpoint threshold of >0.20. | |
| ● | All patients treated with MAT2203 who completed the induction phase achieved sterile CSF cultures during treatment (either during induction or early consolidation phases). | |
| ● | There was no evidence of breakthrough or relapsed cryptococcal infections observed in any of the patients during treatment with MAT2203 through 10 weeks. |
Survival
| ● | Overall survival was 95% in 40 patients randomized to receive MAT2203. |
2*Clin Infect Dis. 2020;71(5):e45-49
| 15 |
Safety
| ● | MAT2203 showed no evidence of renal toxicity or electrolyte abnormalities attributable to MAT2203, no major safety signals, and no use-limiting tolerability issues, even with longer-term treatment with MAT2203 extended beyond induction into the consolidation phase, from week 2 to week 6. |
In Cohort 4 of EnACT, we reported the following results:
Efficacy and EFA
| ● | The CSF yeast clearance rate exceeded the prespecified primary endpoint threshold target of >0.20, with a mean EFA achieved of 0.30 log10 CFU/mL/day with 95% confidence intervals from 0.22 – 0.38. | |
| ● | Several participants with high baseline fungal burdens had noteworthy antifungal activity within the MAT2203 treatment arm, including one patient with quantitative cryptococcal culture as high as 915,000 CFU/mL at the time of screening with effective clearance during the induction period, a key demonstration of potent antifungal activity, even in the most challenging of cases. |
Survival
| ● | In 40 patients receiving MAT2203 treatment, interim survival is currently 90%, while the survival rate at Week 2 was 95%. |
Safety
| ● | MAT2203 patients had fewer Grade ≥3 Clinical Adverse Events (42%) vs. SOC treatment (59%). | |
| ● | The incidence of adverse events relating to kidney function and anemia were significantly lower for MAT2203 compared with the SOC treatment, with no evidence of kidney toxicity seen with six weeks of oral MAT2203 treatment. | |
| ● | The favorable safety and tolerability data seen in Cohort 4 support the use of oral MAT2203 for longer-term use, something not previously feasible due to associated toxicities with currently available IV formulations of amphotericin B. |
The EnACT trial results were published in the Journal of Clinical Infectious Diseases in August 2023 as an Editor’s Choice manuscript.
Expanded/Compassionate Use Access Program
Following completion of the EnACT Trial, we initiated our Expanded/Compassionate Use Access Program in 2022 to demonstrate that MAT2203 could have a significant impact in treating patients with invasive fungal infections with limited or no treatment options, which are very similar to the patients we believed we would be enrolling in a Phase 3 clinical trial.
19 patients to date have been enrolled in the Program at multiple healthcare institutions, including the University of Michigan, Johns Hopkins, Nationwide Children’s Hospital, City of Hope, Vanderbilt University Medical Center, the National Institutes of Health, Children’s Hospital of Philadelphia, Memorial Sloan Kettering Cancer Center, and the University of California, San Diego School of Medicine. The majority of enrolled patients are post-transplant or are undergoing treatment for underlying malignancies. The infections being treated with MAT2203 include a variety of micro-organisms (including Aspergillus, Mucorales species, Candidiasis, Fusarium and suspected Coccidioides) occurring at multiple sites of infection, including brain, bladder/colon, bone, lung, sinus, and skin. Most patients were receiving AmBisome® prior to enrollment but developed treatment-limiting nephrotoxicity and most also required treatment for either azole-resistant organisms or had clinically failed azole therapy and had no other treatment options.
Of the 19 patients enrolled in the Program, 15 have available follow-up and 4 recently initiated or will soon commence treatment with MAT2203.
| 16 |
| ● | 12 of the 15 patients had either complete clinical resolution or objective improvement in clinical markers of infection (including radiologic and mycologic). | |
| ● | Of the 5 patients who completed their full individually specified course of treatment (ranging from 2 weeks to 1 year, depending on the infection) - all have had complete clinical resolution with no relapses or recurrence of their infection. | |
| ● | 5 additional patients have shown objective improvement in clinical markers and are continuing treatment with MAT2203 as planned. | |
| ● | 5 of the 15 patients were unable to complete their individually specified course of treatment, although 2 saw significant clinical improvement of their fungal infection and are included in the 12 overall successful cases. |
| – | 2 patients transitioned to palliative care shortly after starting therapy with MAT2203 because of unanticipated progression of their malignant disease. | |
| – | 1 patient discontinued therapy after two days due to underlying GI issues (i.e., Crohn’s disease). | |
| – | 1 patient passed away due to progression of their underlying disease approximately 8 weeks into therapy (but previously experienced significant clinical improvement of their fungal infection). | |
| – | 1 patient discontinued MAT2203 treatment following 10 weeks of therapy due to underlying GI issues (long-standing nausea/vomiting), but with improvement in their fungal infection. |
Importantly, all patients who experienced renal toxicity following treatment with AmBisome saw their renal function return to baseline after transitioning to MAT2203 therapy and suffered no further renal side effects over the course of extended treatment with MAT2203.
We plan to continue to evaluate opportunities to provide MAT2203 on a compassionate use basis for patients as we believe these are opportunities to showcase the safety and efficacy of MAT2203 outside clinical trial settings which represent important additional patient data for both FDA and prospective partners to review.
The ORALTO Phase 3 Trial
In February 2024, the Company announced that it has reached agreement with the FDA on the design of a the ORALTO trial, including consensus on all critical elements of the registrational path for MAT2203.
ORALTO is a Phase 3, randomized, multicenter, open-label, adjudicator-blinded study to evaluate the efficacy and safety of MAT2203 as an oral step-down treatment following treatment with AmBisome (liposomal IV-amphotericin B) compared with the standard of care in patients with invasive aspergillosis who have limited treatment options. The primary efficacy endpoint is all-cause mortality at study day 42. Key secondary objectives include:
| ● | demonstration of superiority of oral-step down treatment with MAT2203 compared with AmBisome for treatment-related toxicities leading to changes in treatment (i.e., dose adjustment/discontinuations or changes to treatment regimens); |
| ● | long-term survival benefit of MAT2203 using all-cause mortality at study day 84; and |
| ● | evaluation of the impact of MAT2203 on healthcare resource utilization and quality of life impact. |
Enrollment is expected to include approximately 216 adults with recently diagnosed probable or proven invasive aspergillosis who are being treated with AmBisome due to their inability to receive an IV mold-active azole and with limited alternative treatment options. Following up to two days of initial treatment with AmBisome, eligible study participants will be entered into the study and randomized in a 2:1 ratio to receive either oral MAT2203 or continued AmBisome treatment followed by standard of care.
| 17 |
All study participants will receive up to 12 weeks of treatment starting from the first day of treatment with AmBisome. It is anticipated that all study participants will be hospitalized during the initial AmBisome treatment period. After step-down to oral MAT2203, study participants may be discharged from the hospital to continue treatment on an outpatient basis, as clinically appropriate.
An independent Data Review Committee, who will be blinded to treatment, will adjudicate primary and secondary endpoints, including clinical, radiological, and mycological responses. Once approximately 75% of participants are enrolled, an independent Data Safety Monitoring Board will review the overall pooled all-cause mortality rate in a blinded fashion to ensure that the sample size assumptions are reasonable and that the study is adequately powered. Should the pooled event differ substantially from expected levels, a sample size adjustment can be made to the trial.
ORALTO will be conducted at approximately 65 investigator sites in the U.S., Europe, South America, Middle East, and Asia Pacific. Enrollment is expected to commence in the second half of 2024 and is expected to require approximately 24 months.
We are actively engaged in an ongoing partnership process for MAT2203, seeking one or more development and/or commercialization partners. We will require either (i) the consummation of a partnership transaction, or (ii) raising additional capital, prior to commencing the ORALTO trial.
Based on the data generated in Cohorts 2 and 4 of EnACT, data from our ongoing Expanded Access Program, and following our reaching agreement with the FDA on the design of the ORALTO Phase 3 trial design, we believe we have a well-defined pathway to NDA submission for MAT2203 for an initial indication for the treatment of invasive aspergillosis in patients with limited treatment options. Our overall development strategy is to expand the indications for MAT2203 to include the treatment of other IFIs and even prophylaxis once a pharmacodynamic bridge to IV amphotericin has been established through the planned ORALTO Trial.
Overall Clinical Data Package
Clinical studies conducted to evaluate MAT2203 include two completed Phase 1 studies in healthy volunteers (Study CAM-102 and Study MB-70011), and three Phase 2-like studies: one completed study in patients with moderate to severe vulvovaginal candidiasis (VVC), one Phase 2a study in patients with mucocutaneous candidiasis who are refractory or intolerant to standard non-IV therapies, and one Phase 2 study in patients with cryptococcal meningitis.
As of March 2024, MAT2203 has been administered to a total of 202 subjects in four clinical trials and through our Expanded/Compassionate Use Access Program as follows:
| ● | 52 healthy subjects (Studies CAM-102 [N=36] and MB-70011 [N=16]) | |
| ● | 36 patients with HIV and 101 patients with CM (EnACT study MB-70007), | |
| ● | 91 patients with VVC (MB-70005) | |
| ● | 4 patients with mucocutaneous (esophageal and oropharyngeal) candidiasis (MB-70004) | |
| ● | 19 patients in our Expanded/Compassionate Use Access Program in patients |
In these studies, single doses of MAT2203 up to 2.0 g and repeated doses of MAT2203 up to 2.0 g/day and as long as 48 months have been safe and well-tolerated.
About Invasive Aspergillosis in Patients with Limited Treatment Options
Invasive aspergillosis (IA) is a serious and life-threatening invasive fungal infection that occurs primarily in severely immunocompromised patients with hematological malignancies and in transplant recipients. IA has been increasing globally due to advancements in the medical management of these patients and has recently been recognized as a global public health concern. In 2022, the World Health Organization released their Fungal Priority Pathogen List that designated Aspergillus fumigatus, the most common cause of IA, to be in the Critical Priority group (i.e., the highest perceived public health threat). Aspergillus is also included in the FDA qualified designation list of pathogens that pose a serious and life-threatening risk.
| 18 |
The one-year mortality for patients with IA has been reported to be 41% in solid organ transplant recipients and up to 75% in stem cell transplant recipients. Although outcomes have improved since the development of mold-active azoles, the use of these agents is often complicated by treatment-limiting toxicities, drug-drug interactions, and the recent emergence of drug resistance.
The Infectious Diseases Society of America (IDSA) Treatment Guidelines recommend that patients with IA receive treatment with a mold-active azole for a minimum of 6 to 12 weeks, largely dependent on the degree and duration of immunosuppression, site of disease, and evidence of disease. Although the mold-active azoles are generally effective, their use requires a considerable degree of expertise to manage toxicities and drug-drug interactions which often limit duration of treatment.
IA caused by Aspergillus species that are resistant to mold-active azoles has been increasing globally due to the chronic use of azoles and the widespread use of azole fungicides in agriculture. This emerging resistance is particularly concerning because IA due to azole-resistant Aspergillus is a life-threatening disease with a high mortality rate.
Patients with hematological malignancies and transplant recipients often receive antifungal prophylaxis with a mold-active azole to prevent infection during high-risk periods. Recently, cases of breakthrough IA have been reported in patients receiving antifungal prophylaxis. The reason for these apparent failures of prophylaxis may be non-compliance, poor absorption, drug-drug interactions, or infection with a drug-resistant Aspergillus species.
The IDSA Treatment Guidelines recommend an intravenously administered amphotericin B, such as AmBisome, as an alternative for patients with IA who are unable to receive treatment with a mold-active azole. However, IV amphotericin B can cause nephrotoxicity and electrolyte abnormalities which usually requires hospitalization for close monitoring and electrolyte supplementation. Other complications of IV amphotericin B include acute infusion reactions with dyspnea, hypoxia, chest and back pain, IV-site phlebitis, anemia, and hepatotoxicity. As such, treatment with IV amphotericin B for more than a few weeks is generally not safe or practical. There are no oral antifungals available that will enable these patients to complete the recommended 6 to 12 weeks of treatment. Accordingly, there is a critical unmet medical need for effective and well-tolerated oral antifungal agents to treat these patients with IA.
MAT2203 Regulatory Designations
The FDA has granted MAT2203 designations for Qualified Infectious Disease Product, or QIDP, and Fast Track for the treatment of invasive candidiasis and aspergillosis, for the prevention of IFIs in patients on immunosuppressive therapy, and the treatment of cryptococcosis. We recently also received Orphan Drug Designation for MAT2203 for the treatment of cryptococcosis and associated CM from the U.S. FDA and EMA. The FDA may designate a product candidate as an orphan drug if it is intended to treat a rare disease or condition, which is generally defined as having a patient population of fewer than 200,000 individuals in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. The orphan drug designation provides eligibility for orphan drug exclusivity in the United States upon FDA approval if a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation. For a product that obtains orphan drug designation based on a plausible hypothesis that it is clinically superior to the same drug that is already approved for the same indication, to obtain orphan drug exclusivity upon approval, clinical superiority of such product to this same drug that is already approved for the same orphan indication must be demonstrated. Orphan drug exclusivity means that the FDA may not approve any other applications, including a NDA, to market the same drug for the same indication for seven years, except in limited circumstances such as if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Similarly, the FDA can subsequently approve a drug with the same active moiety for the same condition during the exclusivity period if the FDA concludes that the later drug is clinically superior, meaning the later drug is safer, more effective or makes a major contribution to patient care. Orphan drug designation also entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, a waiver from payment of user fees, an exemption from performing clinical studies in pediatric patients unless the FDA requires otherwise by regulation, and tax credits for the cost of the clinical research.
| 19 |
The QIDP designation, provided under the Generating Antibiotic Incentives Now Act, or the GAIN Act, offers certain incentives for the development of new antibacterial or antifungal drugs, including eligibility for Fast Track designation, priority review and, if approved by the FDA, eligibility for an additional five years of marketing exclusivity. Fast Track designation enables more frequent interactions with FDA to expedite drug development and review. Fast Track designation does not change the standards for approval, and we can provide no assurances that we can maintain Fast Track designation for MAT2203 or that such designation will result in faster regulatory review. The seven-year period of marketing exclusivity provided through orphan designation, if granted, combined with an additional five years of marketing exclusivity provided by the QIDP designation positions MAT2203 with a potential for a total of 12 years of marketing exclusivity in the United States to be granted at the time of FDA approval.
Antifungal Market Opportunity
The overall global antifungal market was valued at approximately $15.8 billion in 2023 and is expected to reach approximately $20.5 billion by 2030. In 2021, the global invasive fungal infection market was valued at more than $7.21 billion and is expected to reach US $ 10.36 billion in 2030. This includes therapies used as active treatment or prophylaxis (preventative) in the inpatient and outpatient setting, therapies used for the treatment of hospitalized patients and therapies used for the treatment of patients who are being discharged from the hospital. Importantly, private insurance costs per visit range from approximately $40k to $150K per patient (2019 Benedict) mostly due to extended length of stay. We estimate that, each year, there are over 1.5 million cases of IFIs caused by various species of Candida, Aspergillus and Cryptococcus, the three most common invasive fungal pathogens, globally. The estimated incidence in the U.S. for these conditions is approximately 46,000 for invasive candidiasis, 15,000 for invasive aspergillosis, and 4,900 for CM. For example, aspergillosis-associated hospitalizations in the U.S. alone came at an estimated treatment cost of more than $1.3 billion, with indirect costs amounting to an additional $485 million. The rapid progression of disease and high mortality rates (20% - 50%) associated with documented IFIs often result in antifungal therapy being administered in suspected (unconfirmed) cases or as a preventative measure in patients at high risk. Also, the increasingly widespread use of immune suppressive drugs as cancer chemotherapy or for organ transplantation or treatment of autoimmune disease has resulted in an increasing population of patients at risk for IFIs. Furthermore, the limited number of systemic antifungal drug classes, consisting of azoles, echinocandins and polyenes, and their extensive use, has led to increased numbers of infections with drug-resistant strains. The Centers for Disease Control and Prevention (CDC) has listed fluconazole-resistant Candida as a serious threat requiring prompt and sustained action and has also identified a rise in echinocandin resistance, especially among Candida glabrata. In 2022, the WHO issued a fungal priority pathogens list including cryptococcal neoformans, aspergillus fumigatus and c. auris and c. albicans as critical priority for antifungal development due to the high unmet need. We believe this underscores the urgent need for new agents with demonstrated activity against resistant strains and that can be administered with significantly less toxicity and the potential to discharge patients earlier to reduce hospital stays and associated costs.
LNC Platform Expansion
In addition to advancing MAT2203 into the ORALTO Phase 3 trial, we continue to expand the utilization of the LNC platform with small molecules and small oligonucleotides outside of infectious disease, targeting inflammation and oncology.
Inflammation
We are investigating a variety of LNC formulations of two small oligonucleotides designed to target inflammatory cytokines IL-17A and TNFα and have conducted a series of in vitro and in vivo studies evaluating the biological activity associated with oral delivery as well as the corresponding associated clinical benefit of IL-17A knockdown in an imiquimod (IMQ) induced murine psoriasis disease model.
These preliminary studies have documented biological activity in the form of cytokine knockdown and have also provided some evidence of associated tangible clinical benefit with improvements in skin lesion appearance (redness, scaling) in this qualitative psoriasis model. These data remain under evaluation and the analyses are focused on (a) clarifying the strength and time course of cytokine inhibition, and (b) evaluating cytokine mRNA levels and specific tissue responses in these models to better understand and interpret these data.
| 20 |
In December 2023, we announced results from a series of in vivo studies demonstrating successful oral delivery of two LNC-formulated small single-strand oligonucleotides that specifically target key inflammatory cytokines TNFα and IL-17A in well-established and validated animal models that mimic acute inflammatory responses seen in human diseases.
Acute Colitis Study (“TNFα”)
A dextran sulfate sodium (“DSS”)-induced murine colitis model was used to evaluate an orally administered LNC-delivered small oligonucleotide that specifically targets TNFα mRNA synthesis. Colon tissue TNFα mRNA levels, as assessed by quantitative real-time PCR analysis, were lower following orally administered active LNCs, resulting in statistically significant reductions of serum TNFα levels by 37% compared with diseased, but untreated animals. Importantly, clinical disease activity scores at key time points in the studies were also significantly improved with an active LNC formulation.
Acute Psoriasis Study (“IL-17A”)
An imiquimod (“IMQ”)-induced murine psoriasis model was used to evaluate an orally administered, LNC-delivered small oligonucleotide designed to inhibit IL-17A mRNA synthesis, which contributes significantly to the progression of psoriatic skin lesions. Similar to the DSS colitis model, skin tissue levels of IL-17A mRNA in the IMQ psoriasis model were lower with orally administered active LNCs compared with IMQ alone. In this model, while IL-17A serum levels were not expected to change, improvement was demonstrated in clinical disease markers of skin redness and scaling, further validating the biological activity of these small oligonucleotides.
Oncology
In November of 2023 we announced positive results from an in vivo animal study of an oral LNC formulation of docetaxel, a well-known chemotherapeutic agent used in the management of multiple metastatic and unresectable tumors. Currently, docetaxel is administered intravenously and can be associated with significant side effects and toxicities. The study demonstrated reductions in tumor size comparable to systemic intravenous docetaxel in a well-validated mouse melanoma model. No toxicity was observed with the LNC formulations over the 10 days of oral dosing. The course of treatment primarily targeted efficacy and requires further study and evaluation with longer periods of administration.
Anti-tumor effects of daily oral LNC-docetaxel were comparable to IV-docetaxel with statistically significant reductions in tumor volume compared with untreated controls at Day 14 (high dose oral LNC -63%; low dose oral LNC -57%; IV docetaxel -68%), and similar reductions in tumor weight at Day 14. No systemic toxicities were noted. Body weight was stable over treatment duration and hematologic parameters were similar to untreated controls.
In March 2024 we announced positive results from an additional in vivo study of LNC-docetaxel in healthy mice. The goal of the study was to determine whether an oral LNC formulation of docetaxel could improve the overall safety profile of conventional IV-administered docetaxel. The study included 24 healthy BALB/c mice divided into three treatment groups: (1) control animals treated with oral saline, (2) IV-docetaxel (30 mg/kg, or 0.6 mg/dose) administered once a week for three weeks, and (3) oral LNC-docetaxel (37.5 mg/kg, or 0.75 mg/dose) administered once daily over three weeks. The primary endpoint of the study was change in body weight over the treatment period which how toxicity is primarily manifested in this model.
Key takeaways included the following:
| ● | Through Day 22, the total amount of docetaxel administered with the oral LNC-docetaxel formulation was more than 8x greater than with IV-docetaxel (the final IV dose was used to measure pharmacokinetics). | |
| ● | All mice treated with IV-docetaxel lost a significant amount of weight (toxicity), with an average peak loss of 20% of their original body weight. | |
| ● | Mice treated with oral LNC-docetaxel maintained their body weight, which was statistically no different than the weight of control mice treated with oral saline. |
| 21 |
| ● | One mouse in the IV-docetaxel group died prior to the conclusion of the study and was censored from the analysis (last measurement was -32% body weight loss); the curve of the IV-docetaxel group also reflects the expected 7-day recovery following an IV dose of docetaxel. |
The daily administered oral LNC-docetaxel dose was 50% higher, and the total amount of drug administered was 3.5 times greater, than the LNC-docetaxel dose administered in a previous study that demonstrated anti-tumor activity comparable to IV-docetaxel in a syngeneic mouse melanoma model.
Next steps include evaluating the efficacy of the current LNC-docetaxel formulation in other tumor models. Additionally, we plan to evaluate the potential anti-tumor activity of LNC formulations of small oligonucleotides.
Strategic Collaborations Involving the LNC Platform
We continue to believe that our LNC Platform can be used to reformulate a wide variety of molecules and drugs which (i) require delivery technology to effectively protect molecules and drugs in the body and could benefit from efficient delivery and cellular uptake by target cells, and (ii) are currently only available in IV formulations or, (iii) otherwise experience significant toxicity-related AEs. We have tested a range of pharmaceutical compounds reformulated by using our LNC Platform in proof-of-concept animal studies, including oligonucleotides (mRNA, siRNA, DNA plasmids), vaccines, anti-inflammatory agents, NSAIDs and atovaquone. We intend to pursue opportunities to develop products, either alone or in partnership with other pharmaceutical or biotech companies, and this remains a key part of our strategy to maximize the value of this unique and disruptive LNC Platform.
In December 2019, we announced a feasibility collaboration with Genentech, a Roche company, to evaluate formulations of several Genentech compounds utilizing our LNC Platform. The original agreement provided for cooperation on up to three proprietary Genentech compounds for initial in vitro testing. Two of the programs have been completed. Each demonstrated the successful intracellular delivery of LNC-formulated small molecules and oligonucleotides, without accompanying toxicity. We have chosen to slow this collaboration to focus on our internal small oligonucleotide programs in inflammation and our small molecule programs in oncology.
In December 2020, we announced a collaboration with the NIAID to develop oral formulations of Gilead’s remdesivir, which is currently only available as an intravenous therapy in the fight against COVID-19. During 2021 and 2022, the NIAID, together with the Department of Epidemiology at the University of North Carolina at Chapel Hill, conducted two in vivo tests of our LNC formulation of Gilead Science’s remdesivir (LNC-RDV) in a standard genetically modified mouse model of SARS-CoV-2 infection. In these animal models, orally administered LNC-RDV reduced viral titers and improved clinical parameters of body weight and congestion scores five days following infection, with effects similar to those seen with subcutaneous administered remdesivir. Following discussion and review of the data with Gilead, the Company was informed that Gilead would focus on its internally developed prodrugs of remdesivir given their advanced clinical placement.
In April 2022, we, along with BioNTech SE, a global pharmaceutical company focused on mRNA technologies, announced an exclusive research collaboration to evaluate the combination of BioNTech’s mRNA formats with our proprietary LNC Platform. Under the terms of the agreement with BioNTech, we received an exclusivity fee in the amount of $2.75 million, and BioNTech funded certain of our research expenses related to the collaboration. We collaborated closely on formulation, optimization, and in vitro testing and a single in vivo study was conducted in 2023. The in vivo study of oral mRNA delivery did not demonstrate oral preclinical activity. This formulation successfully delivered mRNA in vitro in multiple cell lines prior to advancing to the in vivo study in healthy mice. Additional internal in vivo studies of similar non-lipid nanocrystal (“LNC”) mRNA formulations did show activity when administered systemically (intramuscularly and intraperitoneally). In addition, these formulations demonstrated a high degree of stability to at least 17 weeks at 4° Celsius which compares favorably to lipid nanoparticles (“LNPs”); The research collaboration between Matinas and BioNTech concluded in May of 2023 following the in vivo study results.
In January 2023, we entered into a Material Transfer and Evaluation Agreement with National Resilience, Inc. (Resilience) focused on exploring the potential for oral delivery of identified nucleic acids. We have informed Resilience that we have prioritized our internal work in small oligonucleotides but could revisit this collaboration in the future.
| 22 |
We continue to evaluate additional potential strategic collaborations with other interested biotechnology and pharmaceutical partners. These early stage, proof-of-concept evaluations could provide an efficient, less expensive pathway to create numerous strategic verticals in areas of innovative medicine while capitalizing on the development expertise and financial resources of well-established partners. Data from these evaluations could position us as a licensor of our LNC Platform to numerous strategic partners better positioned to absorb the risks and costs of drug development while allowing our company to become a royalty aggregator with the potential to generate upfront license, milestone, and royalty payments as we maximize the value of the overall LNC Platform.
Exclusive License Agreement with Rutgers University
Through our acquisition of Aquarius Biotechnologies Inc., we acquired a license from Rutgers for certain patents related to the LNC Platform. We subsequently changed the name of Aquarius Biotechnologies Inc. to Matinas BioPharma Nanotechnologies, Inc. and in February of 2022, the parties agreed to a Second Amended and Restated Exclusive License Agreement. The agreement provides for (1) royalties on a tiered basis between low single digits and the mid-single digits of net sales of products using such licensed technology, (2) a one-time sales milestone fee of $100,000 when and if sales of products using the licensed technology reach the specified sales threshold and (3) an annual license fee of $50,000 over the term of the license agreement. There was also a reduction in the consideration paid to Rutgers in the event of a sublicense to a third party of the exclusive patent rights granted pursuant to the Agreement. In consideration of the concessions made by Rutgers in the amended license agreement, the Company issued Rutgers 400,000 shares of common stock in February 2022. We also agreed to continue to assume the responsibility to pay required patent prosecution and maintenance fees covering the technology.
Unless otherwise terminated by either party, the term of the license, on a country-by-country basis, shall be the longer of 8-1/2 years from the date of first commercial sale of a product in a country using the licensed technology or until the expiration of the last-to-expire patent rights licensed under the agreement, whichever is longer. Rutgers has the right to terminate the license agreement if we have not commenced commercial sales of at least one product using the licensed technology within eight years of the effective date of the Second Amended and Restated License Agreement.
Intellectual Property
The proprietary nature of, and protection for, our product candidates and our discovery programs, processes and know-how are important to our business. We will seek to protect our products and associated technologies for their manufacturing and development through a combination of patents, trade secrets, proprietary know-how, FDA exclusivity and contractual restrictions on disclosure. Our policy is to pursue, maintain and defend patent rights and to protect the technology, inventions and improvements that are commercially important to the development of our business. Our success will significantly depend on our ability to obtain and maintain patent and other proprietary protection for commercially important technology and inventions and know-how related to our business, defend and enforce our patents, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely heavily on know-how and continuing technological innovation to develop and maintain our proprietary position.
Exclusively Licensed and Matinas-Owned Intellectual Property Relating to Our Proprietary LNC Platform and MAT2203
The patents and patent applications that we exclusively license from Rutgers provide some patent protection for the proprietary chemistry technology used in certain of our processes to make our lipid nanocrystal and geodate cochleates and formulate the active pharmaceutical ingredients delivered inside this delivery technology, as in MAT2203, our lead product comprising the LNC Platform. Pursuant to our license agreement, we acquired rights to a portfolio that as of December 31, 2023 included 1 pending U.S. non-provisional patent application, 9 U.S. patents, and 49 granted foreign patents, which extends patent protection until at least 2033, excluding patent term adjustments or extensions. The in-licensed patents have been granted in countries including Europe, China, India, Brazil, Russia, Canada, Japan, Korea, Australia and Mexico.
The Matinas-owned patent portfolio covers our LNC Platform and users. This patent portfolio includes 5 pending U.S. provisional applications, 2 pending U.S. non-provisional applications, 2 pending PCT applications, 13 pending foreign applications, and 20 granted foreign patents. The foreign pending applications and granted patents are in countries including Europe, China, Brazil, Canada, Japan, Korea, Australia and Mexico
| 23 |
As of December 31, 2023, we owned one issued U.S. patent, 7 issued foreign patents in Australia, Canada, Europe, and Japan, and one pending foreign patent application in Korea, directed to compositions and methods for enhancing tissue penetration of an active agent in an LNC. The patents are expected to expire in 2036, and patents issuing from or claiming priority to the pending application are also expected to expire in 2036, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned 9 foreign patents in Australia, Europe, and Japan, directed to LNC compositions and methods for treating mycobacteria infection. The patents are expected to expire in 2036, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one U.S. issued patent, one pending non-provisional U.S. patent application, 2 issued foreign patents in Japan and Australia, and 4 pending applications in China, Korea, Canada, and Europe, directed to LNC compositions and methods for treating cryptococcus infections. The patents are expected to expire in 2037, and patents issuing from or claiming priority to the pending application are also expected to expire in 2037, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one pending non-provisional U.S. patent application, and 4 pending applications in Australia, Brazil, Canada, China, Europe, Hong Kong, Japan, and Mexico, directed to LNC compositions and methods for treating cryptococcus infections. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2040, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one PCT application directed to methods for controlling LNC particle size. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2043, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one PCT application directed to LNC compositions and methods for treating mucormycosis. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2043, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned three U.S. provisional applications directed to LNC platform improvements for delivery of therapeutic agents, including small oligonucleotides. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2044, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one U.S. provisional application directed to lipid particle formulations for nucleic acid delivery. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2044, excluding patent term adjustments or extensions.
As of December 31, 2023, we owned one U.S. provisional application directed to LNC compositions and methods for treating cancer. Patents issuing from or claiming priority to the pending applications are also expected to expire in 2044, excluding patent term adjustments or extensions.
We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications we may own or license in the future, nor can we be sure that any of our existing patents or any patents we may own or license in the future will be useful in protecting our technology. For this and more comprehensive risks related to our intellectual property, please see “Risk Factors—Risks Relating to Our Intellectual Property and Regulatory Exclusivity.”
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of our proprietary LNC Platform are based on unpatented trade secrets and know-how. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
| 24 |
We also plan to seek trademark protection in the United States and outside of the United States where available and when appropriate. We intend to use these registered marks in connection with our pharmaceutical research and development as well as our product candidates.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. We face competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions. Many of these companies have far greater human and financial resources and may have product candidates in more advanced stages of development and many will reach the market before our product candidates. Competitors may also develop products that are more effective, safer or less expensive or that have better tolerability or convenience.
Although we believe that our proprietary LNC Platform, experience, and knowledge in our areas of focus provide us with competitive advantages, potential competitors could reduce our commercial opportunities. For many of our product candidates, we anticipate facing competition from other products that are available on a generic basis and offered at low prices. Many of these generic products have been marketed by third parties for many years and are well accepted by physicians, patients, and payers.
We believe that MAT2203 and any other development candidate we may pursue in the future using our proprietary LNC Platform, paralleled with our scientific and development expertise in the field of drug delivery, provide us with competitive advantages over our peers. However, we face potential competition from various sources, including larger and better-funded pharmaceutical, specialty pharmaceutical, and biotechnology companies, as well as from generic drug manufacturers, academic institutions, governmental agencies, and public and private research institutions.
MAT2203 will primarily compete with antifungal classes approved for the treatment of fungal and mold infections, which include polyenes, azoles and echinocandins. The approved branded therapies for these indications include Cancidas (caspofungin, marketed by Merck & Co.), Eraxis (anidulafungin, marketed by Pfizer, Inc.), Mycamine (micafungin, marketed by Astellas Pharma US, Inc.), Diflucan (fluconazole, marketed by Pfizer, Inc.), Noxafil (posaconazole, marketed by Merck & Co.), Vfend (voriconazole, marketed by Pfizer, Inc.), Sporanox (itraconazole, marketed by Jansen Pharmaceuticals, Inc.), Cresemba (isavuconazole, marketed by Astellas Pharma US, Inc.), Ambisome (liposomal amphotericin B, marketed by Astellas Pharma US, Inc.) Abelcet (lipid complex amphotericin B, marketed by Sigma Tau Pharmaceuticals Inc.), Rezzayo (rezafungin, marketed by Melinta Therapeutics), Brexafemme (Ibrexafungerp marketed by GlaxoSmithKline) and amphotericin B deoxycholate (marketed by X-Gen Pharmaceuticals, Inc.). There currently are and may be more generic versions of these products available at the time of MAT2203 market approval, which will create added competition. In addition to approved therapies, we expect that MAT2203 may compete with product candidates that we are aware of in clinical development by third parties, such olorofim (being developed by F2G, Ltd), fosmanogepix (being developed by Basilea), and AM2-19, a derivative of amphotericin B being developed by Elion Therapeutics.
Manufacturing
We currently lease and operate in-house manufacturing capabilities for our lead LNC Platform product candidate, MAT2203, and for our LNC Platform discovery programs in the small molecule and small oligonucleotide spaces. While sufficient to produce the clinical supplies of product necessary to conduct our ongoing clinical trials and potentially early commercialization of MAT2203, we are exploring relationships with well-respected third-party contract manufacturers for the formulation and manufacture of MAT2203 necessary to support the filing of an NDA and to support commercial manufacture for this product. We may also need to expand our internal manufacturing capabilities in the future. If we are not able to retain our current manufacturing facilities and if we do not develop additional in-house manufacturing capability for MAT2203 and our other product candidates sufficient to produce product for commercialization of these products, we will need to develop relationships with third-party manufacturers for the manufacture of our product candidates which could be time consuming and expensive.
| 25 |
In the first quarter of 2022, we selected and reached agreement with Thermo Fisher Scientific to support scale-up and manufacturing for MAT2203 in anticipation of a potential NDA submission. Thermo Fisher Scientific, with more than 65 locations around the world, provides integrated, end-to-end capabilities across all phases of development, including APIs, biologics, viral vectors, cGMP plasmids, formulation, clinical trials solutions, logistics services and commercial manufacturing and packaging. During 2022 we worked with Thermo Fisher to prepare for a technology transfer of certain of the processes we use in the formulation and manufacture of MAT2203. As part of our decision to prioritize the regulatory feedback from FDA on our plan for a Phase 3 trial and our desire to secure a pharmaceutical or governmental partner to advance the development of MAT2203 into Phase 3, we slowed down our transition to Thermo Fisher and our planned related expenses during 2023. We anticipate the resumption of activities with Thermo Fisher once additional financing from one or more of these sources has been secured.
There are several potential third-party suppliers for amphotericin B, the generic active pharmaceutical ingredient in our lead clinical stage product candidate – MAT2203. Although to date we have not entered into formal supply agreements to secure sufficient supply of amphotericin B to support our clinical programs for MAT2203, we believe we will be able to secure supply of amphotericin B to support our clinical programs for MAT2203 from one or more third-party suppliers. As we move through development for our product candidates, we expect to enter into long-term supply arrangements for key active pharmaceutical ingredients.
Sales and Marketing
We currently do not have any sales and marketing infrastructure. We plan to retain U.S. marketing and sales rights or co-promotion rights for our product candidates for which we receive marketing approvals, particularly in situations where it is possible to access the market through a focused, specialized sales force. For situations in which a large sales force is required to access the market, and with respect to markets outside the United States, we generally plan to commercialize our product candidates through collaborative arrangements with leading pharmaceutical and biotechnology companies.
Review and Approval of Drugs in the United States
In the United States, FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant and/or sponsor to a variety of administrative or judicial sanctions, including refusal by FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by FDA and the Department of Justice (DOJ) or other governmental entities.
Our product candidates must be approved by FDA through the NDA or biologics license application (BLA), in the case of biologic product candidates, process before they may be legally marketed in the United States. An applicant seeking approval to market and distribute a new drug product in the United States must typically undertake the following:
| ● | completion of nonclinical laboratory tests, animal studies and formulation studies in compliance with FDA’s good laboratory practice (cGLP), regulations; |
| ● | submission to FDA of an investigational new drug applications (IND), which must take effect before human clinical trials may begin; |
| 26 |
| ● | approval by an independent institutional review board (IRB) representing each clinical site before each clinical trial may be initiated; |
| ● | performance of adequate and well-controlled human clinical trials in accordance with current good clinical practices (GCP), to establish the safety and efficacy of the proposed drug product for each indication; |
| ● | preparation and submission to FDA of an NDA or BLA; |
| ● | review of the product by an FDA advisory committee, where appropriate or if applicable; |
| ● | satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices (cGMP), requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| ● | payment of user fees and securing FDA approval of the NDA or BLA; and |
| ● | compliance with any post-approval requirements, including a risk evaluation and mitigation strategy (REMS), and post-approval studies required by FDA. |
Nonclinical Studies
Nonclinical studies include laboratory evaluation of the purity and stability of the manufactured drug substance or active pharmaceutical ingredient and the formulated drug or drug product, as well as in vitro and animal studies to assess the safety and activity of the drug for initial testing in humans and to establish a rationale for therapeutic use. The conduct of nonclinical studies is subject to federal regulations and requirements, including cGLP regulations. The results of the nonclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, are submitted to FDA as part of an IND.
Companies usually must complete some long-term nonclinical testing, such as animal tests of reproductive AEs and carcinogenicity, and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the drug in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
Human Clinical Trials in Support of a Regulatory Approval
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to FDA as part of the IND. An IND automatically becomes effective 30 days after receipt by FDA, unless before that time FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and FDA must resolve any outstanding concerns before the clinical trial can begin. Accordingly, submission of an IND may or may not result in FDA allowing clinical trials to commence.
In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health for public dissemination on their ClinicalTrials.gov website.
| 27 |
A sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. When a foreign clinical study is conducted under an IND, all FDA IND requirements must be met unless waived. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to FDA in support of an NDA or IND so long as the clinical trial is conducted in accordance with GCP and if FDA is able to validate the data from the clinical trial through an on-site inspection if FDA deems it necessary.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The drug is initially introduced into a small number of healthy human subjects or patients with the target disease (e.g. cancer) or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
Phase 2: The drug is administered to a larger number of trial participants, up to several hundred, who usually have the disease or condition that the experimental drug is intended to treat, to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: These clinical trials are commonly referred to as “pivotal” studies, which typically denotes a study which presents the data that FDA or another relevant regulatory agency will use to determine whether or not to approve a drug. In Phase 3 clinical trials, the drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Progress reports detailing the results of the clinical trials must be submitted at least annually to FDA and more frequently if serious AEs occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. FDA will typically inspect one or more clinical sites to assure compliance with GCP and the integrity of the clinical data submitted.
Submission of an NDA to FDA
Regulatory approval for most new drug or biologic products is based on two adequate and well-controlled Phase 3 clinical trials that provide evidence of the safety and efficacy of the proposed new product. Assuming successful completion of required clinical testing and other requirements, the results of the nonclinical and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls, and proposed labeling, among other things, are submitted to FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to an application user fee and the sponsor of an approved NDA is also subject to annual prescription drug program fees and establishment user fees. These fees are typically increased annually.
FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after FDA’s receipt of the submission whether the application is sufficiently complete to permit substantive review. FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before FDA accepts it for filing. Once the submission is accepted for filing, FDA begins an in-depth substantive review. FDA has agreed to specified performance goals in the review process of NDAs. Most such applications are meant to be reviewed within ten months from the date of filing, and most applications for “priority review” products are meant to be reviewed within six months of filing. The review process may be extended by FDA for various reasons, and for various time periods, including for three additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by FDA following the original submission.
| 28 |
Before approving an NDA, FDA typically will inspect the facility or facilities where the product is or will be manufactured. These pre-approval inspections cover all facilities associated with an NDA submission, including drug component manufacturing (such as Active Pharmaceutical Ingredients), finished drug product manufacturing and control testing laboratories. FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, FDA will typically inspect one or more clinical sites to assure compliance with GCP.
The FDA is required to refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Fast Track, Breakthrough Therapy and Priority Review Designations
FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are fast track designation, breakthrough therapy designation and priority review designation.
Specifically, FDA may designate a product for Fast Track review if it is intended, whether alone or in combination with one or more other drugs, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For Fast Track products, sponsors may have greater interactions with FDA and FDA may initiate review of sections of a fast-track product’s NDA before the application is complete. This rolling review may be available if FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a Fast Track product may be effective. The sponsor must also provide, and FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, FDA’s time goal for reviewing a Fast Track application does not begin until the last section of the NDA is submitted. In addition, the Fast Track designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Second, in 2012, Congress enacted the Food and Drug Administration Safety and Improvement Act, or FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as “breakthrough therapies.” A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
Third, FDA may designate a product for priority review if it is a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. FDA determines, on a case-by-case basis, whether the proposed drug represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten FDA’s goal for taking action on a marketing application from ten months to six months.
| 29 |
Under Section 524 of the FDCA, the FDA is authorized to award a priority review voucher to sponsors of certain tropical disease product applications that meet the criteria specified in the Act. A priority review voucher may be used by the sponsor who obtains it, or it may be transferred to another sponsor who may use it to obtain priority review for a different application. Priority review vouchers can result in the acceleration of review and approval of a product candidate by up to four months. In order to be eligible for a tropical disease priority review voucher, the application must be: for a listed tropical disease; submitted under Section 505(b)(1) of the FDCA or Section 351 of the Public Health Service Act; for a product that contains no active ingredient that has been approved in any other application under those statutory provisions; and must qualify for priority review. FDA has identified in guidance those product applications for the prevention or treatment of tropical diseases that may qualify for a priority review voucher.
Accelerated Approval Pathway
FDA may grant accelerated approval to a drug for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the drug has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. FDA may also grant accelerated approval for such a condition when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Drugs granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a drug, such as an effect on IMM. FDA has limited experience with accelerated approvals based on intermediate clinical endpoints but has indicated that such endpoints generally may support accelerated approval where the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate clinical benefit of a drug.
The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a drug, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the drug’s clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, would allow FDA to withdraw the drug from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by FDA.
FDA’s Decision on an NDA
Based on FDA’s evaluation of the NDA and accompanying information, including the results of the inspection of the manufacturing facilities, FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information for FDA to reconsider the application. When those deficiencies have been addressed to FDA’s satisfaction in a resubmission of the NDA, FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
| 30 |
If FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions which can materially affect the potential market and profitability of the product. In addition, as a condition of approval, FDA may require an applicant to develop a REMS. REMS use risk minimization strategies beyond the professional labeling to ensure that the benefits of the product outweigh the potential risks. To determine whether a REMS is needed, FDA will consider the size of the population likely to use the product, seriousness of the disease, expected benefit of the product, expected duration of treatment, seriousness of known or potential AEs and whether the product is a new molecular entity. REMS can include medication guides, physician communication plans for healthcare professionals and elements to assure safe use, which may include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. FDA may require a REMS before approval or post-approval if it becomes aware of a serious risk associated with use of the product. The requirement for a REMS can materially affect the potential market and profitability of a product.
FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with FDA and state agencies and are subject to periodic unannounced inspections by FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning or untitled letters or holds on post-approval clinical trials; |
| ● | refusal of FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
| 31 |
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Abbreviated New Drug Applications (ANDA) for Generic Drugs
In 1984, with passage of the Hatch-Waxman Amendments to the FDCA, Congress authorized FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application (ANDA), to the agency. In support of such applications, a generic manufacturer may rely on the nonclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference listed drug (RLD).
Specifically, for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form and the strength of the drug. At the same time, the FDA must also determine that the generic drug is “bioequivalent” to the innovator drug. Under the statute, a generic drug is bioequivalent to a RLD if the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug.
Upon approval of an ANDA, the FDA indicates whether the generic product is therapeutically equivalent to the RLD in its publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” also referred to as the “Orange Book.” Physicians and pharmacists consider a therapeutically equivalent generic drug to be fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, FDA’s designation of therapeutic equivalence often results in automatic substitution of the generic drug by the pharmacist without the knowledge or consent of either the prescribing physician or patient.
Under the Hatch-Waxman Amendments, the FDA may not approve an ANDA until any applicable period of non-patent exclusivity for the RLD has expired. The FDCA provides a period of five years of non-patent data exclusivity for a new drug containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA may not be submitted to FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication.
Hatch-Waxman Patent Certification and the 30 Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant submits its application to the FDA, the applicant is required to certify to FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. Specifically, the applicant must certify with respect to each patent that:
| ● | the required patent information has not been filed; |
| ● | the listed patent has expired; |
| ● | the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or |
| 32 |
| ● | the listed patent is invalid, unenforceable or will not be infringed by the new product. |
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, an NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in 2012, sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA and the FDA’s internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent exclusivity. This six-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application.
Orphan Designation and Exclusivity
Under the Orphan Drug Act, the FDA may designate a drug product as an “orphan drug” if it is intended to treat a rare disease or condition (generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a drug product available in the United States for treatment of the disease or condition will be recovered from sales of the product). A company must request orphan product designation before submitting a NDA. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan product designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
If a product with orphan status receives the first the FDA approval for the disease or condition for which it has such designation, the product will be entitled to orphan product exclusivity. Orphan product exclusivity means that the FDA may not approve any other applications for the same product for the same indication for seven years, except in certain limited circumstances. Competitors may receive approval of different products for the indication for which the orphan product has exclusivity and may obtain approval for the same product but for a different indication. If a drug or drug product designated as an orphan product ultimately receives marketing approval for an indication broader than what was designated in its orphan product application, it may not be entitled to exclusivity.
| 33 |
21st Century Cures Act
On December 13, 2016, Congress passed the 21st Century Cures Act, or the Cures Act. The Cures Act is designed to modernize and personalize healthcare, spur innovation and research, and streamline the discovery and development of new therapies through increased federal funding of particular programs. It authorizes increased funding for the FDA to spend on innovation projects. The new law also amends the Public Health Service Act to reauthorize and expand funding for the National Institutes of Health. The Act establishes the NIH Innovation Fund to pay for the cost of development and implementation of a strategic plan, early-stage investigators and research. It also charges NIH with leading and coordinating expanded pediatric research. In addition, the Cures Act includes provisions requiring the FDA to assess and publish guidance on the use of novel clinical trial designs, the use of real-world evidence in applications, the availability of summary level review for supplemental applications for certain indications, and the qualification of drug development tools. Because the Cures Act has only recently been enacted, its potential effect on our business remains unclear with the exception of a provision requiring that we post our policies on the availability of expanded access programs for individuals. Because these provisions allow the FDA to spend several years developing these policies, the effect on us could be delayed.
With amendments to the FDCA and the Public Health Service Act, or PHSA, Title III of the Cures Act seeks to accelerate the discovery, development, and delivery of new medicines and medical technologies. To that end, and among other provisions, the Cures Act reauthorizes the existing priority review voucher program for certain drugs intended to treat rare pediatric diseases until 2026; creates a new priority review voucher program for drug applications determined to be material national security threat medical countermeasure applications; and revises the FDCA to streamline review of combination product applications.
Section 3042 of the Cures Act authorizes a “Limited Population Pathway” to expedite approval of antimicrobial products intended to treat serious or life-threatening infections for which there are unmet medical needs. Drugs approved under this provision are required to adhere to special labeling requirements, including a prominent “Limited Population” statement. We will monitor the implementation of the Cures Act but cannot currently assess how this initiative may impact our business.
Other Health Care Regulations
Health Privacy Laws
We are subject to data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the FTC Act), govern the collection, use, disclosure, and protection of health-related and other personal information. Failure to comply with data protection laws and regulations could result in government enforcement actions and create liability for us (which could include civil and/or criminal penalties), private litigation and/or adverse publicity that could negatively affect our operating results and business. In addition, we may obtain health information from third parties (e.g., principal investigators involved in our clinical trials) that are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, or HIPPA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH. HIPAA generally requires that covered entities (healthcare providers, health plans and healthcare clearinghouses) obtain written authorizations from patients prior to disclosing protected health information of the patient (unless an exception to the authorization requirement applies). If authorization is required and the patient fails to execute an authorization or the authorization fails to contain all required provisions, then we may not be allowed access to and use of the patient’s information and our research efforts could be impaired or delayed. Furthermore, use of protected health information that is provided to us pursuant to a valid patient authorization is subject to the limits set forth in the authorization (e.g., for use in research and in submissions to regulatory authorities for product approvals). Among other things, HITECH makes HIPAA’s privacy and security standards, as well as the various penalties or failure to comply, directly applicable to “business associates”—independent contractors or agents of covered entities performing certain functions involving the creation or use of protected health information on behalf of a covered entity or providing services to a covered entity. While we do not believe we are a “business associate” under HIPAA, regulatory agencies may disagree.
| 34 |
The General Data Protection Regulation, or GDPR, adopted in 2016, establishes a regulatory framework designed to protect the security of personal data collected about residents of the EU and the movement of such personal data across the national borders of the EU Member States, including, but not limited to, requirements to obtaining consent of the individuals to whom the personal data relates, the nature and scope of notifications provided to the individuals, the security and confidentiality of the personal data, data breach notification and using third party processors in connection with the processing of the personal data. Failure to comply with the EU Directive and the GDPR could subject us to regulatory sanctions, delays in clinical trials, criminal prosecution and/or civil fines or penalties. Additionally, GDPR creates a direct cause of action by individual data subjects.
Fraud and Abuse Laws
In addition to the FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal prosecution, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback statute and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Affordable Care Act
In late March 2010, the Federal government enacted the comprehensive health care reform package, the Affordable Care Act (ACA). Among other provisions, the ACA imposes individual and employer health insurance requirements, provides certain insurance subsidies (e.g., premiums and cost sharing), mandates extensive insurance market reforms, creates new health insurance access points (e.g., State and federal-based health insurance exchanges), expands the Medicaid program, promotes research on comparative clinical effectiveness of different technologies and procedures, and makes a number of changes to how products and services will be reimbursed by the Medicare program. Amendments to the Federal False Claims Act under the ACA have made it easier for private parties to bring “qui tam” (whistleblower) lawsuits against companies, under which the whistleblower may be entitled to receive a percentage of any money paid to the government.
Since its enactment, there have been judicial and Congressional challenges and amendments to certain aspects of the ACA. There is continued uncertainty about the implementation of the ACA, including the potential for further amendments to the ACA and legal challenges to or efforts to repeal the ACA. If the ACA is repealed or further modified, or if implementation of certain aspects of the ACA are delayed, such repeal, modification or delay may materially adversely impact our business, strategies, prospects, operating results or financial condition. We are unable to predict the full impact of any repeal, modification or delay in the implementation of the ACA on us at this time. Due to the substantial regulatory changes that will need to be implemented by CMS and others, and the numerous processes required to implement these reforms, we cannot predict which healthcare initiatives will be implemented at the federal or state level, the timing of any such reforms, or the effect such reforms or any other future legislation or regulation will have on our business.
| 35 |
Designation of and Exclusivity for Qualified Infectious Disease Products
In 2012, Congress passed legislation known as the Generating Antibiotic Incentives Now Act, or GAIN Act. This legislation is designed to encourage the development of antibacterial and antifungal drug products that treat pathogens that cause serious and life-threatening infections. To that end, the law grants an additional five years of marketing exclusivity upon the approval of an NDA for a drug product designated by FDA as a Qualified Infectious Disease Product, or QIDP. Thus, for a QIDP, the periods of five-year new chemical entity exclusivity, three-year new clinical investigation exclusivity and seven-year orphan drug exclusivity, would become 10 years, eight years, and 12 years, respectively.
A QIDP is defined in the GAIN Act to mean “an antibacterial or antifungal drug for human use intended to treat serious or life-threatening infections, including those caused by—(1) an antibacterial or antifungal resistant pathogen, including novel or emerging infectious pathogens;” or (2) certain “qualifying pathogens.” A “qualifying pathogen” is a pathogen that has the potential to pose a serious threat to public health (e.g., resistant gram-positive pathogens, multi-drug resistant gram-negative bacteria, multi-drug resistant tuberculosis and Clostridium difficile) and that is included in a list established and maintained by FDA. A drug sponsor may request FDA to designate its product as a QIDP any time before the submission of an NDA. FDA must make a QIDP determination within 60 days of the designation request. A product designated as a QIDP will be granted priority review by FDA and can qualify for “fast track” status.
The additional five years of market exclusivity under the GAIN Act for drug products designated by FDA as QIDPs applies only to a drug that is first approved on or after July 9, 2012. Additionally, the five-year exclusivity extension does not apply to: a supplement to an application under Section 505(b) of the FDCA for any QIDP for which an extension is in effect or has expired; a subsequent application submitted with respect to a product approved by FDA for a change that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device or strength; or a product that does not meet the definition of a QIDP under Section 505(g) based upon its approved uses.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, which permits a patent restoration of up to five years for patent term lost during product development and FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of a NDA, plus the time between the submission date of a NDA and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drugs for which approval is sought can only be extended in connection with one of the approvals. The USPTO reviews and approves the application for any patent term extension or restoration in consultation with FDA.
Review and Approval of Drug Products in the European Union
To market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of drug products. Whether or not it obtains FDA approval for a product, the company would need to obtain the necessary approvals by the comparable non-U.S. regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
| 36 |
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of a European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. Clinical trial application must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a drug under European Union regulatory systems, an applicant must submit a marketing authorization application, or MAA, either under a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or the CHMP, established at the European Medicines Agency, or EMA, is responsible for conducting the initial assessment of a drug. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
The decentralized procedure is available to applicants who wish to market a product in various European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one-member state designated by the applicant, known as the reference member state. Under this procedure, an applicant applies based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, whose decision is binding on all member states.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by FDA and other government authorities. Sales of products will depend, in part, on the extent to which the costs of the products will be covered by third party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication. Additionally, the containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results.
| 37 |
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Third party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product, or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
Healthcare Law and Regulation
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with third party payors and customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations. Such restrictions under applicable federal and state healthcare laws and regulations, include the following:
| ● | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| ● | the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| ● | the HIPPA imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| ● | HIPAA, as amended by the HITECH and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| 38 |
| ● | the federal transparency requirements under the ACA requires manufacturers of drugs to report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests and the reported information will be made publicly available on a searchable website; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Human Capital Resources
As of March 18, 2024, we had 32 full-time employees. There are no collective bargaining agreements covering any of our employees.
We believe that our success depends on our ability to attract, develop and retain key personnel. We believe that the skills, experience and industry knowledge of our key employees significantly benefit our operations and performance.
Employee health and safety in the workplace is one of our core values and we provide a safe and secure workplace for our employees.
Employee levels are managed to align with the pace of business and management believes it has sufficient human capital to operate its business successfully.
Research and Development
For the years ended December 31, 2023 and 2022, we incurred $14,489 and $16,678, respectively, on research and development activities. These expenses include cash and non-cash expenses relating to the development of our clinical and pre-clinical programs, including our anti-infective product candidates, MAT2203 and MAT2501 as well as support and enhancement of our LNC Platform. Our research and development expenses reflect the reimbursement of certain MAT2501 program expenses of $88 thousand and $811 thousand during the years ended December 31, 2023 and 2022, respectively, related to the CFF grant agreement.
Corporate and Available Information
We were incorporated in Delaware under the name Matinas BioPharma Holdings, Inc. in May 2013. We have two operating subsidiaries: Matinas BioPharma, Inc., a Delaware corporation originally formed on August 12, 2011 as Nereus BioPharma LLC, and Matinas BioPharma Nanotechnologies, Inc., a Delaware corporation originally formed on January 29, 2015 as Aquarius Biotechnologies, Inc.
Our principal executive offices are located at 1545 Route 206 South, Suite 302, Bedminster, New Jersey 07921, and our telephone number is (908) 484-8805. Our website address is www.matinasbiopharma.com. Our website and the information contained on, or that can be accessed through, our website will not be deemed to be incorporated by reference into this Annual Report on Form 10-K or any other report we file with or furnish to the SEC.
We make available on our website, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or the SEC. Our SEC reports can be accessed through the Investors section of our internet website. Further, a copy of this Annual Report on Form 10-K is located at the SEC’s Public Reference Rooms at 100 F Street, N.E., Washington, D. C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at http://www.sec.gov.
| 39 |
| Item 1A. | Risk Factors |
An investment in our common stock is speculative and involves a high degree of risk, including a risk of loss of your entire investment. You should carefully consider the risks described below and the other information in this Annual Report before purchasing shares of our common stock. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties may also adversely impair our business operations. If any of the events described in the risk factors below actually occur, our business, financial condition or results of operations could suffer significantly. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock.
Summary of Risk Factors
| ● | We have incurred significant losses since our inception. We expect to incur losses over the next several years and may never achieve or maintain profitability. |
| ● | We will need substantial additional funding. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts. |
| ● | Raising additional capital may cause dilution to stockholders, restrict operations or require us to relinquish rights to our technologies or product candidates. |
| ● | Our stockholders may be subject to substantial dilution by exercises of outstanding options and warrants. |
| ● | Our operating history to date may make it difficult to evaluate the success of our business and assess our future viability. |
| ● | We are early in our development efforts, which may not be successful. |
| ● | We cannot be certain that our product candidates will receive regulatory approval, without which we will not be able to market any of our product candidates. Any delay in the approval process will harm our business. |
| ● | We depend in part on technology owned or licensed to us by third parties, the loss of which would terminate or delay the further development of our product candidates, injure our reputation, or force us to pay higher royalties. |
| ● | Clinical drug development involves a lengthy and expensive process and uncertain as to outcome. |
| ● | Delays in any aspect of our clinical trials could result in increased costs to us and delay or limit our ability to obtain regulatory approval for our product candidates. |
| ● | We may not have or be able to obtain sufficient quantities of our products to meet our supply and clinical studies obligations and our business, financial condition and results of operation may be adversely affected. |
| ● | If we are unable to successfully commercialize our products our ability to generate revenue will be limited. |
| ● | If our preclinical and clinical studies do not produce positive results, if our clinical trials are delayed or if serious side effects are identified during such studies or trials, we may experience delays, incur additional costs and ultimately be unable to commercialize our product candidates. |
| ● | If we cannot enroll enough patients to complete our clinical trials, our business, financial condition and results of operations may be adversely affected. |
| ● | We may not be able to maintain orphan drug designation or exclusivity for our anti-infective product candidates. |
| ● | Any Fast Track designation or grant of priority review status by the FDA may not actually lead to a faster development or regulatory review or approval process, nor will it assure FDA approval of our product candidates. Additionally, our product candidates may treat indications that do not qualify for priority review vouchers. |
| ● | Any breakthrough therapy designation granted by the FDA for our product candidates may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that our product candidates will receive marketing approval. |
| ● | Designation of our product candidates as qualified infectious disease products is not assured and, in any event, even if granted, may not actually lead to a faster development or regulatory review, and would not assure FDA approval of our product candidates. |
| 40 |
| ● | We may not be able to obtain or maintain orphan drug designation, Fast Track designation, qualified infection disease designation or breakthrough therapy designation for any of our product candidates, and even if granted, such designations may not actually lead to a faster development or regulatory review and would not assure FDA approval of any of our product candidates. |
| ● | If we are unsuccessful in identifying and developing additional product candidates, our potential for growth may be impaired. |
| ● | We currently have no sales and marketing organization. If we are unable to establish satisfactory sales and marketing capabilities, we may not successfully commercialize any of our product candidates, even if regulatory approval is obtained. |
| ● | If we are unable to file for approval of MAT2203 under Section 505(b)(2) of the FDCA or if we are required to generate additional data related to safety and efficacy in order to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines. |
| ● | We face competition from other biotechnology and pharmaceutical companies and our operating results will suffer if we fail to compete effectively. |
| ● | Even if we obtain marketing approval for any product candidate, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates could be subject to labeling and other restrictions and withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our future products. |
| ● | Future legislation, and/or regulations and policies adopted by the FDA may increase the time and cost required for us to conduct and complete clinical trials. |
| ● | Changes in health care law and implementing regulations may have a material adverse effect on us. |
| ● | Our future growth depends, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties. |
| ● | If we market our product candidates in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties. |
| ● | We have been and expect to be significantly dependent on our collaborative agreements for the development of MAT2203, which exposes us to the risk of reliance on the performance of third parties. |
| ● | We expect that we will rely on third parties to conduct clinical trials for our product candidates, which exposes us to the risk of reliance on the performance of third parties. |
| ● | We are, and will be, completely dependent on third parties to manufacture our product candidates, and our commercialization efforts could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of any product candidate or fail to do so at acceptable quality levels or prices. |
| ● | Unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives could harm our business. |
| ● | Outbreaks of communicable diseases may materially and adversely affect our business, financial condition and results of operations. |
| ● | Adverse global conditions, including economic uncertainty, may negatively impact our financial results. |
| ● | We depend on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from discovering, developing and commercializing product candidates. |
| ● | It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. |
| ● | If we fail to obtain or maintain patent or trade secret protection for our technologies, third parties could use our proprietary information, which could impair our ability to compete in the market and adversely affect our ability to generate revenues and attain profitability. |
| ● | Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts. |
| ● | We will need to increase the size of our organization to grow our business, and we may experience difficulties in managing this growth. |
| ● | If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. In addition, the loss of the services of certain key employees would adversely impact our business prospects. |
| ● | If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. |
| 41 |
| ● | Our internal computer systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs. |
| ● | We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions. |
| ● | Pursuant to the terms of our Series A Preferred Stock, we may be obligated to pay significant royalties. |
| ● | The rights of the holders of common stock may be impaired by the potential issuance of preferred stock. |
| ● | We do not intend to pay dividends on our common stock in the foreseeable future. |
| ● | An active public trading market for our common stock may not be sustained. |
| ● | Our share price has been and could remain volatile. |
| ● | If securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline. |
| ● | We could be delisted from the NYSE American, which could seriously harm the liquidity of our stock and our ability to raise capital. |
| ● | Upon dissolution of our Company, you may not recoup all or any portion of your investment. |
| ● | Our certificate of incorporation allows for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock. |
| ● | Anti-takeover provisions of our certificate of incorporation, bylaws and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management. |
| ● | Stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees could be limited. |
| ● | Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. |
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since our inception. We expect to incur losses over the next several years and may never achieve or maintain profitability.
We have incurred significant operating losses in every year since inception and expect to incur net operating losses for the foreseeable future. Our net loss was $22,942 thousand and $20,997 thousand for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $175,573 thousand. We do not know whether or when we will become profitable. To date, we have not generated any revenues from product sales and have financed our operations through private placements and public offerings of our equity securities and, to a lesser extent, through funding from the Cystic Fibrosis Foundation, or CFF, and the National Institutes of Health, or the NIH. We have devoted substantially all our financial resources and efforts to the research and development of potential product candidates. All our product candidates are in the development stage, and we have not completed development of any product candidate. We expect to continue to incur significant expenses and operating losses over the next several years. Our net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ deficit and working capital. We anticipate that our expenses will increase substantially if and as we:
| ● | conduct further clinical and preclinical studies of MAT2203, our lead LNC product candidate; |
| ● | support the conduct of further clinical studies of MAT2203, even if such studies are partially financed with non-dilutive funding from the NIH; |
| ● | seek to discover and develop additional product candidates; |
| ● | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| ● | require the manufacture of larger quantities of product candidates for clinical development and potentially commercialization; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| 42 |
| ● | hire additional clinical, quality control and scientific personnel; and |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts and personnel and infrastructure necessary to help us comply with our obligations as a public company. |
Our ability to become and remain profitable depends on our ability to generate revenue. We do not expect to generate significant revenue until we are able to obtain marketing approval for, and successfully commercialize, one or more of our product candidates. This will require us to be successful in a range of challenging activities, including completing preclinical testing and clinical trials of our product candidates, discovering additional product candidates, obtaining regulatory approval for these product candidates, manufacturing, marketing, and selling any products for which we may obtain regulatory approval, satisfying any post-marketing requirements and obtaining reimbursement for our products from private insurance or government payors. We are only in the preliminary stages of most of these activities and have not yet commenced other of these activities. We may never succeed in these activities and, even if we do, may never generate revenues that are significant enough to achieve profitability.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. If we are required by the U.S. Food and Drug Administration, or the FDA, or comparable non-U.S. regulatory authorities to perform studies in addition to those currently expected, or if there are any delays in completing our clinical trials or the development of any of our product candidates, our expenses could increase.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our pipeline of product candidates, or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we could be forced to delay, reduce, or eliminate our product development programs or commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we conduct additional clinical studies of our product candidates, including the potential Phase 3 clinical trials of MAT2203, and conduct additional preclinical and clinical trials to further validate and expand our LNC Platform, continue research and development, initiate clinical trials and, if development succeeds, seek regulatory approval of our product candidates. Our expenses could further increase if we initiate new research and preclinical development efforts for other product candidates. In addition, if we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales, and distribution. Furthermore, we expect to incur significant additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce, or eliminate our research and development programs or any future commercialization efforts.
We believe that our existing cash, cash equivalents and marketable debt securities, excluding restricted cash, of $13,756 thousand as of December 31, 2023 will enable us to fund our operating expenses and capital expenditure requirements into the third quarter of 2024, but not beyond. We have based this estimate on assumptions that may prove to be wrong in the future, and we could use our capital resources sooner than we currently expect. Changing circumstances could cause us to consume capital significantly faster than we currently anticipate, and we may need to spend more money than currently expected because of circumstances beyond our control. Our future capital requirements, both short-term and long-term, will depend on many factors, including:
| ● | the progress, timing, costs, and results of our ongoing and planned clinical trials of our product candidates; |
| ● | the scope, progress, timing, costs, and results of clinical trials of, and research and preclinical development efforts for, other product candidates, including MAT2203, any future product candidates based upon our LNC Platform, and any preclinical or clinical work done to further validate our LNC Platform, generally; |
| 43 |
| ● | our ability to enter into and the terms and timing of any collaborations, licensing or other arrangements that we may establish; |
| ● | the number and development requirements of other product candidates that we pursue; |
| ● | the costs, timing, and outcome of regulatory review of our product candidates by the FDA and comparable non-U.S. regulatory authorities; |
| ● | the costs and timing of future commercialization activities, including product manufacturing, marketing, sales, and distribution, for any of our product candidates for which we receive marketing approval; |
| ● | the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval; |
| ● | our headcount growth and associated costs as we expand our research and development and establish a commercial infrastructure; |
| ● | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; |
| ● | the extent to which we acquire or in-license other products and technologies; |
| ● | the costs of operating as a public company; and |
| ● | the effect of competing technological and market developments. |
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive, and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate product revenues sufficient to achieve profitability, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, government or other third-party funding, collaborations and licensing arrangements. We do not have any committed external source of funds other than limited grant funding from the NIH. To the extent that we raise additional capital through the sale of common stock, convertible securities or other equity securities, your ownership interest may be materially diluted, and the terms of these securities may include liquidation or other preferences and anti-dilution protections that could adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends, that could adversely impact our ability to conduct our business. Securing additional financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development of our product candidates.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
| 44 |
Our stockholders may be subject to substantial dilution by exercises of outstanding options.
As of December 31, 2023, we had outstanding options to purchase an aggregate of 46,707,934 shares of our common stock at a weighted average exercise price of $0.83 per share. The exercise of such outstanding options will result in dilution of the value of our shares.
Our operating history to date may make it difficult to evaluate the success of our business and to assess our future viability.
We commenced active operations in 2013 and our product candidates are in early stages of clinical development. We have not yet demonstrated our ability to successfully obtain regulatory approvals for any of our product candidates, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays, and other known and unknown factors. Even if we obtain regulatory approval, we will need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter-to-quarter and year-to-year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
We are early in our development efforts, which may not be successful.
Because we are still in the clinical stage of our development efforts and are in the process of determining the overall clinical development path for our current and future product candidates, the timing and costs of the regulatory paths we will follow. Our ability to generate product revenue, which we do not expect will occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of our product candidates. The success of MAT2203 and any other product candidates we may develop will depend on many factors, including the following:
| ● | successful completion of preclinical studies; |
| ● | successful enrollment in, and completion of, clinical trials: |
| ● | demonstrating safety and efficacy; |
| ● | receipt of marketing approvals from applicable regulatory authorities; |
| ● | establishing clinical and commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
| ● | obtaining and maintaining patent and trade secret protection and non-patent exclusivity for our product candidates and technologies; |
| ● | launching commercial sales of the product candidates, if approved, whether alone or selectively in collaboration with others; |
| 45 |
| ● | acceptance of the product candidates, if approved, by patients, the medical community and third-party payers; |
| ● | effectively competing with other therapies; |
| ● | a continued acceptable safety profile of the products following approval; and |
| ● | enforcing and defending intellectual property rights and claims. |
If we do not accomplish one or more of these goals in a timely manner, or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would harm our business.
We cannot be certain that any of our product candidates will receive regulatory approval, without which we will not be able to market any of our product candidates. Any delay in the approval process will harm our business.
We expect to invest most of our capital in the development of our LNC Platform. Our ability to generate revenue related to product sales, which we do not expect will occur for at least the next several years, if ever, will depend on the successful development and regulatory approval of one or more of our product candidates. All our product candidates require regulatory review and approval prior to commercialization. Any delays in the regulatory review or approval of our product candidates would delay market launch, increase our cash requirements and result in additional operating losses. This failure to obtain regulatory approvals would prevent our product candidate from being marketed and would have a material and adverse effect on our business.
The process of obtaining FDA and other required regulatory approvals, including foreign approvals, often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. Furthermore, this approval process is extremely complex, expensive, and uncertain. We may be unable to submit any NDA in the United States or any marketing approval application in foreign jurisdictions for any of our products. If we submit an NDA including any amended NDA or supplemental NDA, to the FDA seeking marketing approval for any of our product candidates, the FDA must decide whether to accept or reject the submission for filing. We cannot be certain that any of these submissions will be accepted for filing and reviewed by the FDA, or that the marketing approval application submissions to any other regulatory authorities will be accepted for filing and review by those authorities. We cannot be certain that we will be able to respond to any regulatory requests during the review period in a timely manner, or at all, without delaying potential regulatory action. We also cannot be certain that any of our product candidates will receive favorable recommendations from any FDA advisory committee or foreign regulatory bodies or be approved for marketing by the FDA or foreign regulatory authorities. In addition, delays in approvals or rejections of marketing applications may be based upon many factors, including regulatory requests for additional analyses, reports, data and studies, regulatory questions regarding data and results, changes in regulatory policy during the period of product development and the emergence of new information regarding such product candidates.
Data obtained from preclinical studies and clinical trials are subject to different interpretations, which could delay, limit, or prevent regulatory review or approval of any of our product candidates. Furthermore, regulatory attitudes towards the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, policy changes and agency funding, staffing and leadership. We do not know whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects.
In addition, the environment in which our regulatory submissions may be reviewed changes over time. For example, average review times at the FDA for NDAs have fluctuated in recent years, and we cannot predict the review time for any of our submissions with any regulatory authorities. Review times can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes. Moreover, considering widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of the U.S. Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and establishment of REMS measures that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before completion or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or may result in approval for a more limited indication than originally sought.
| 46 |
We depend in part on technology owned or licensed to us by third parties, the loss of which would terminate or delay the further development of our product candidates, injure our reputation, or force us to pay higher royalties.
We rely heavily on the LNC Platform and certain of the patents that we have exclusively licensed from Rutgers. The loss of access to these patents could materially impair our business and future viability, and could result in delays in developing, introducing, or maintaining our product candidates and formulations until equivalent technology, if available, is identified, licensed and integrated. In addition, any defects in the intellectual property that we license could prevent the implementation or impair the functionality of our product candidates or formulation, delay new product or formulation introductions or injure our reputation. If we are required to enter into license agreements with third parties for replacement technology, we could be subject to higher royalty payments.
We may not have or be able to obtain sufficient quantities of our products to meet our supply and clinical studies obligations and our business, financial condition and results of operation may be adversely affected.
To date, we have only developed limited in-house manufacturing capabilities for the LNC Platform needed for the clinical development our MAT2203 product candidates. We have entered into an agreement with Patheon, a wholly owned subsidiary of ThermoFisher, to prepare for the commercial manufacture of MAT2203. If we do not develop a long-term manufacturing capability for our LNC platform product candidates sufficient to produce product for continued development and, if regulatory approval is obtained, then commercialization of these products, we will be dependent on a small number of third-party manufacturers for the manufacture of our product candidates. We may not have long-term agreements with any of these third parties, and if they are unable or unwilling to perform for any reason, we may not be able to locate alternative acceptable manufacturers or formulators or enter into favorable agreements with them. Any inability to acquire enough of our products in a timely manner from these third parties could delay clinical trials and prevent us from developing our products in a cost-effective manner or on a timely basis. In addition, manufacturers of our product candidates are subject to cGMP and similar foreign standards, and we would not have control over compliance with these regulations by our manufacturers. If one of our contract manufacturers fails to maintain compliance, the production of our products could be interrupted, resulting in delays and additional costs. In addition, if the facilities of such manufacturers do not pass a pre-approval or post-approval plant inspection, the FDA will not grant approval and may institute restrictions on the marketing or sale of our products.
We may be reliant on third party manufactures and suppliers to meet the demands of our clinical supplies. Delays in receipt of materials, scheduling, release, custom’s control, and regulatory compliance issues may adversely impact our ability to initiate, maintain, or complete clinical trials that we are sponsoring. Commercial manufacturing and supply agreements have not been established. Issues arising from scale-up, environmental controls, public health crises, such as pandemics and epidemics, equipment requirements, or other factors, may have an adverse impact on our ability to manufacture our product candidates.
If we are unable to successfully commercialize our products our ability to generate revenue will be limited.
Even if we obtain regulatory approval for our product candidates, our long-term viability and growth depend on the successful commercialization of products which lead to revenue and profits. Pharmaceutical product development is an expensive, high risk, lengthy, complicated, resource intensive process. To succeed, among other things, we must be able to:
| ● | identify potential drug product candidates; |
| ● | design and conduct appropriate laboratory, preclinical and other research; |
| ● | submit for and receive regulatory approval to perform clinical studies; |
| ● | design and conduct appropriate preclinical and clinical studies according to good laboratory and good clinical practices; |
| 47 |
| ● | select and recruit clinical investigators; |
| ● | select and recruit subjects for our studies; |
| ● | collect, analyze, and correctly interpret the data from our studies; |
| ● | submit for and receive regulatory approvals for marketing; and |
| ● | manufacture the drug product candidates according to cGMP. |
The development program with respect to any given product will take many years and thus delay our ability to generate profits. In addition, potential products that appear promising at early stages of development may fail for several reasons, including the possibility that the products may require significant additional testing or turn out to be unsafe, ineffective, too difficult or expensive to develop or manufacture, too difficult to administer, or unstable. Failure to successfully commercialize our products will adversely affect our business, financial condition, and results of operations.
If our preclinical and clinical studies do not produce positive results, if our clinical trials are delayed or if serious side effects are identified during such studies or trials, we may experience delays, incur additional costs and ultimately be unable to commercialize our product candidates.
Before obtaining regulatory approval for the sale of our product candidates, we must conduct, generally at our own expense, extensive preclinical tests to demonstrate the safety of our product candidates in animals, and clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Preclinical and clinical testing is expensive, difficult to design and implement and can take many years to complete. A failure of one or more of our preclinical studies or clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to obtain regulatory approval or commercialize our product candidates, including:
| ● | our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials or we may abandon projects that we expect to be promising; |
| ● | regulators or institutional review boards may not authorize us to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| ● | conditions imposed on us by the FDA or any non-U.S. regulatory authority regarding the scope or design of our clinical trials may require us to resubmit our clinical trial protocols to institutional review boards for re-inspection due to changes in the regulatory environment; |
| ● | the number of patients required for our clinical trials may be larger than we anticipate, or participants may drop out of our clinical trials at a higher rate than we anticipate; |
| ● | our third-party contractors or clinical investigators may fail to comply with regulatory requirements or fail to meet their contractual obligations to us in a timely manner; |
| ● | we might have to suspend or terminate one or more of our clinical trials if we, the regulators or the institutional review boards determine that the participants are being exposed to unacceptable health risks; |
| ● | regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| ● | the cost of our clinical trials may be greater than we anticipate; |
| 48 |
| ● | the supply or quality of our product candidates or other materials necessary to conduct our clinical trials may be insufficient or inadequate or we may not be able to reach agreements on acceptable terms with prospective clinical research organizations; and |
| ● | the effects of our product candidates may not be the desired effects or may include undesirable side effects or the product candidates may have other unexpected characteristics. |
In addition, if we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| ● | be delayed in obtaining, or may not be able to obtain, marketing approval for one or more of our product candidates; |
| ● | obtain approval for indications that are not as broad as intended or entirely different than those indications for which we sought approval; or |
| ● | have the product removed from the market after obtaining marketing approval. |
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether any preclinical tests or clinical trials will be initiated as planned, will need to be restructured or will be completed on schedule, if at all. Significant preclinical or clinical trial delays also could shorten the patent protection period during which we may have the exclusive right to commercialize our product candidates. Such delays could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
If we cannot enroll enough patients to complete our clinical trials, our business, financial condition, and results of operations may be adversely affected.
The completion rate of clinical studies of our products is dependent on, among other factors, the patient enrollment rate. Patient enrollment is a function of many factors, including:
| ● | investigator identification and recruitment; |
| ● | regulatory approvals to initiate study sites; |
| ● | patient population size; |
| ● | the nature of the protocol to be used in the trial; |
| ● | patient proximity to clinical sites; |
| ● | eligibility criteria for the study; |
| ● | competition from other companies’ clinical studies for the same patient population; and |
| ● | ability to obtain comparator drug/device. |
We believe our procedures for enrolling patients have been appropriate; however, delays in patient enrollment would increase costs and delay ultimate commercialization and sales, if any, of our products. Such delays could materially adversely affect our business, financial condition, and results of operations.
| 49 |
We may not be able to maintain orphan drug designation or exclusivity for our anti-infective product candidates.
We have received orphan drug designation for MAT2203 in the United States and may seek additional orphan drug designation for other product candidates. Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of regulatory or marketing exclusivity, which precludes the FDA or the EMA from approving another marketing application for the same indication for that drug during that time. For a product that obtains orphan drug designation on the basis of a plausible hypothesis that it is clinically superior to the same drug that is already approved for the same indication, in order to obtain orphan drug exclusivity upon approval, clinical superiority of such product to this same drug that is already approved for the same orphan indication must be demonstrated. The exclusivity period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or the EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
We cannot assure you that the application for orphan drug designation of MAT2203 or any future application with respect to any other product candidate, will be maintained or granted. If we are unable to maintain orphan drug designation in the United States, we will not be eligible to obtain the period of market exclusivity that could result from orphan drug designation or be afforded the financial incentives associated with orphan drug designation. Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
Any Fast Track designation or grant of priority review status by the FDA may not actually lead to a faster development or regulatory review or approval process, nor will it assure FDA approval of our product candidates. Additionally, our product candidates may treat indications that do not qualify for priority review vouchers.
We have received Fast Track designation for MAT2203 for the treatment of invasive candidiasis, the treatment of aspergillosis, the prevention of invasive fungal infections due to immunosuppressive therapy and the treatment of cryptococcosis and may seek Fast Track designation for some of our other product candidates or priority review of applications for approval of our product candidates for certain indications. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA Fast Track designation. If a product candidate offers major advances in treatment, the FDA may designate it eligible for priority review. The FDA has broad discretion whether to grant these designations, so even if we believe a particular product candidate is eligible for these designations, we cannot assure you that the FDA would decide to grant them. Even if we do receive Fast Track designation or priority review, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program.
Any breakthrough therapy designation granted by the FDA for our product candidates may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that our product candidates will receive marketing approval.
We may seek a breakthrough therapy designation for some of our product candidates. A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs and biologics that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs designated as breakthrough therapies by the FDA may also be eligible for accelerated approval if the relevant criteria are met.
| 50 |
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as breakthrough therapies, the FDA may later decide that the products no longer meet the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Designation of our product candidates as qualified infectious disease products is not assured and, in any event, even if granted, may not actually lead to a faster development or regulatory review, and would not assure FDA approval of our product candidates.
We have received a qualified infectious disease product, or QIDP, designation for MAT2203 for certain indications and we may be eligible for designation of future product candidates as QIDPs. A QIDP is “an antibacterial or antifungal drug intended to treat serious or life-threatening infections, including those caused by an antibacterial or antifungal resistant pathogen, including novel or emerging infectious pathogens or certain “qualifying pathogens.” A product designated as a QIDP will be granted priority review by the FDA and may qualify for “fast track” status. Upon the approval of an NDA for a drug product designated by the FDA as a QIDP, the product is granted a period of five years of regulatory exclusivity in addition to any other period of regulatory exclusivity for which the product is eligible. The FDA has broad discretion whether to grant these designations, so even if we believe a particular product candidate is eligible for such designation or status, the FDA could decide not to grant it. Moreover, even if we do receive such a designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures and there is no assurance that our product candidate, even if determined to be a QIDP, will be approved by the FDA.
If we are unsuccessful in identifying and developing additional product candidates, our potential for growth may be impaired.
Even if we receive regulatory approval for MAT2203 or any other product candidates we may develop, we still may not be able to successfully commercialize such products and the revenue that we generate from its sales, if any, may be limited.
If approved for marketing, the commercial success of MAT2203 or any other product candidates we may develop will depend upon its acceptance by the medical community, including physicians, patients, and health care payors. The degree of market acceptance of MAT2203 or such other product candidate will depend on several factors, including:
| ● | demonstration of clinical safety and efficacy of such product candidate; |
| ● | relative convenience and ease of administration; |
| ● | the prevalence and severity of any adverse effects; |
| ● | the willingness of physicians to prescribe such product candidates and of the target patient population to try new therapies; |
| ● | pricing and cost-effectiveness; |
| ● | the inclusion or omission of such product candidate in applicable treatment guidelines; |
| ● | the effectiveness of our or any future collaborators’ sales and marketing strategies; |
| ● | limitations or warnings contained in FDA approved labeling; |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors; and |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement. |
| 51 |
If MAT2203 or any other product candidates we may develop is approved but does not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of such product candidate may require significant resources and may never be successful.
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize such product candidate successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render such product candidate not commercially viable. For example, regulatory authorities may approve such product candidate for fewer or more limited indications than we request, may not approve the price we intend to charge for such product candidate, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve such product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that indication. Further, the FDA may place conditions on approvals including potential requirements or risk management plans and the requirement for a REMS to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS; the FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of such product candidate. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of such product candidate.
We currently have no sales and marketing organization. If we are unable to establish satisfactory sales and marketing capabilities, we may not successfully commercialize any of our product candidates, even if regulatory approval is obtained.
At present, we have no sales or marketing personnel. To commercialize products that are approved for commercial sales, we must either develop a sales and marketing infrastructure or collaborate with third parties that have such commercial infrastructure. If we elect to develop our own sales and marketing organization, we do not intend to begin to hire sales and marketing personnel until the time of NDA submission to the FDA at the earliest, and we do not intend to establish our own sales organization in the United States until shortly prior to FDA approval of MAT2203 or any of our other product candidates.
We may not be able to establish a direct sales force in a cost-effective manner or realize a positive return on this investment. In addition, we will have to compete with established and well-funded pharmaceutical and biotechnology companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize MAT2203 or any of our other product candidates in the United States without strategic partners or licensees include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe our future products; |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
| 52 |
If we are not successful in recruiting sales and marketing personnel or in building a sales and marketing infrastructure, or if we do not successfully enter into appropriate collaboration arrangements, we will have difficulty successfully commercializing MAT2203 or any other product candidates we may develop, which would adversely affect our business, operating results and financial condition. Outside the United States, we may commercialize our product candidates by entering into collaboration agreements with pharmaceutical partners. We may not be able to enter into such agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties.
If we are unable to file for approval of MAT2203 under Section 505(b)(2) of the FDCA or if we are required to generate additional data related to safety and efficacy to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines.
Our current plans for filing the NDAs for MAT2203 include efforts to minimize the data we will be required to generate to obtain marketing approval for this product candidate and therefore reduce the development time. We intend to rely on the history of efficacy of amphotericin B, and although we met with the FDA in 2019, 2021 and again in 2022 to discuss our development plans for MAT2203, there is no assurance we will satisfy FDA’s requirements for approval of MAT2203 under a 505(b)(2) pathway. The timeline for filing and review of our NDA for MAT2203 is based on our plan to submit the NDA under Section 505(b)(2) of the FDCA, which would enable us to rely in part on data in the public domain or elsewhere. We have not yet filed an NDA under Section 505(b)(2) for any product candidate. Depending on the data that may be required by the FDA for approval, some of the data may be related to products already approved by the FDA. If the data relied upon is related to products already approved by the FDA and covered by third-party patents, we would be required to certify that we do not infringe the listed patents or that such patents are invalid or unenforceable. As a result of the certification, the third-party would have 45 days from notification of our certification to initiate an action against us.
If an action is brought in response to such a certification, the approval of our NDA could be subject to a stay of up to 30 months or more while we defend against such a suit. Approval of our product candidates under Section 505(b)(2) may therefore be delayed until patent exclusivity expires or until we successfully challenge the applicability of those patents to our product candidates. Alternatively, we may elect to generate sufficient additional clinical data so that we no longer rely on data which triggers a potential stay of the approval of our product candidates. Even if no exclusivity periods apply to our applications under Section 505(b)(2), the FDA has broad discretion to require us to generate additional data on the safety and efficacy of our product candidates to supplement third-party data on which we may be permitted to rely. In either event, we could be required, before obtaining marketing approval for any of our product candidates, to conduct substantial new research and development activities beyond those we currently plan to engage to obtain approval of our product candidates. Such additional new research and development activities would be costly and time consuming.
We may not be able to realize a shortened development timeline for any of our product candidates, and the FDA may not approve our NDA based on their review of the submitted data. If our desired reference-listed drug containing products are withdrawn from the market by the FDA for any safety reason, we may not be able to reference such products to support a 505(b)(2) NDA for our product candidates, and we may need to fulfill the more extensive requirements of Section 505(b)(1). If we are required to generate additional data to support approval, we may be unable to meet our anticipated development and commercialization timelines, may be unable to generate the additional data at a reasonable cost, or at all, and may be unable to obtain marketing approval of our lead product candidates.
We face competition from other biotechnology and pharmaceutical companies and our operating results will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We have competitors in several jurisdictions, many of which have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than we have. We face competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions. Established competitors may invest heavily to quickly discover and develop novel compounds that could make MAT2203 or any other product candidates we may develop obsolete or uneconomical. Any new product that competes with an approved product may need to demonstrate compelling advantages in efficacy, cost, convenience, tolerability, and safety to be commercially successful. Other competitive factors, including generic competition, which could force us to lower prices or result in reduced sales, particularly those products that have been marketed by third parties for many years and are well accepted by physicians, patients, and payers. In addition, new products developed by others could emerge as competitors to MAT2203 or any of our other product candidates. If we are not able to compete effectively against our current and future competitors, our business will not grow, and our financial condition and operations will suffer.
Further, although we believe that our proprietary LNC Platform, experience, and knowledge in our areas of focus provide us with competitive advantages, potential competitors for MAT2203 could reduce our commercial opportunities.
| 53 |
Even if we obtain marketing approval for any product candidate, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates could be subject to labeling and other restrictions and withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our future products.
Even if we obtain United States regulatory approval of MAT2203 or any other product candidates that we may develop, FDA may still impose significant restrictions on its indicated uses or marketing or the conditions of approval or impose ongoing requirements for potentially costly and time-consuming post-approval studies, and post-market surveillance to monitor safety and efficacy. Our future products will also be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of AEs and other post-market information. These requirements include registration with FDA, as well as continued compliance with current Good Clinical Practices regulations, or cGCPs, for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continuous review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, cGMP, requirements relating to quality control, quality assurance and corresponding maintenance of records and documents.
FDA has the authority to require a REMS, as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria or requiring patient testing, monitoring and/or enrollment in a registry.
With respect to sales and marketing activities by us or any future partner, advertising and promotional materials must comply with FDA rules in addition to other applicable federal, state, and local laws in the United States and similar legal requirements in other countries. In the United States, the distribution of product samples to physicians must comply with the requirements of the U.S. Prescription Drug Marketing Act. Application holders must obtain FDA approval for product and manufacturing changes, depending on the nature of the change. We may also be subject, directly or indirectly through our customers and partners, to various fraud and abuse laws, including, without limitation, the U.S. Anti-Kickback Statute, U.S. False Claims Act, and similar state laws, which impact, among other things, our proposed sales, marketing, and scientific/educational grant programs. If we participate in the U.S. Medicaid Drug Rebate Program, the Federal Supply Schedule of the U.S. Department of Veterans Affairs, or other government drug programs, we will be subject to complex laws and regulations regarding reporting and payment obligations. All of these activities are also potentially subject to U.S. federal and state consumer protection and unfair competition laws. Similar requirements exist in many of these areas in other countries.
In addition, our product labeling, advertising, and promotion would be subject to regulatory requirements and continuing regulatory review. FDA strictly regulates the promotional claims that may be made about prescription products. A product may not be promoted for uses that are not approved by FDA as reflected in the product’s approved labeling. If we receive marketing approval for our product candidates, physicians may nevertheless legally prescribe our products to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability and government fines. FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions, including revocation of its marketing approval. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. FDA has also requested that companies enter into consent decrees of permanent injunctions under which specified promotional conduct is changed or curtailed.
| 54 |
If we or a regulatory agency discovers previously unknown problems with a product, such as AEs of unanticipated severity or frequency, problems with the facility where the product is manufactured, or we or our manufacturers fail to comply with applicable regulatory requirements, we may be subject to the following administrative or judicial sanctions:
| ● | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| ● | issuance of warning letters or untitled letters; |
| ● | clinical holds; |
| ● | injunctions or the imposition of civil or criminal penalties or monetary fines; |
| ● | suspension or withdrawal of regulatory approval; |
| ● | suspension of any ongoing clinical trials; |
| ● | refusal to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| ● | suspension or imposition of restrictions on operations, including costly new manufacturing requirements; or |
| ● | product seizure or detention or refusal to permit the import or export of product. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize MAT2203 or any of our other product candidates and generate revenue. Adverse regulatory action, whether pre- or post-approval, can also potentially lead to product liability claims and increase our product liability exposure.
Future legislation, and/or regulations and policies adopted by the FDA may increase the time and cost required for us to conduct and complete clinical trials.
FDA has established regulations to govern the drug development and approval process, as have foreign regulatory authorities. The policies of FDA and other regulatory authorities may change, and additional laws or government regulations may be promulgated that could prevent, limit, delay but also accelerate regulatory review of our product candidates. For example, in December 2016, the Cures Act was signed into law. The Cures Act, among other things, is intended to modernize the regulation of drugs and spur innovation. We cannot predict what if any effect the Cures Act or any existing or future guidance from FDA will have on development of our product candidates.
Changes in health care law and implementing regulations may have a material adverse effect on us.
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. For example, in the United States, the Patient Protection and Affordable Care Act of 2010 (“ACA”) substantially changed the way health care is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. Many provisions of the ACA impact the biopharmaceutical industry, including that in order for a biopharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the drug pricing program under the Public Health Services Act, or PHS.
| 55 |
Additionally, the Inflation Reduction Act of 2022, which took effect in 2023, includes policies that are designed to have a direct impact on drug prices and reduce drug spending by the federal government. This legislation contains substantial drug pricing reforms, including the establishment of a drug price negotiation program within the U.S. Department of Health and Human Services that would require manufacturers to charge a negotiated “maximum fair price” for certain selected drugs covered by Medicare or pay an excise tax for noncompliance, the establishment of rebate payment requirements on manufacturers of certain drugs payable under Medicare Parts B and D to penalize price increases that outpace inflation, and requires manufacturers to provide discounts on Part D drugs.
Legislative, administrative, and private payor efforts to control drug costs span a range of proposals, including drug price negotiation, Medicare Part D redesign, drug price inflation rebates, international mechanisms, generic drug promotion and anticompetitive behavior, manufacturer reporting, and reforms that could impact therapies utilizing the accelerated approval pathway. We cannot predict the ultimate content, timing or effect of any changes to the ACA, the Inflation Reduction Act, or other federal and state healthcare policy reform efforts including those aimed at drug pricing. There is no assurance that federal or state health care reform will not adversely affect our future business and financial results, and we cannot predict how future federal or state legislative, judicial or administrative changes relating to healthcare policy will affect our business.
We cannot be sure whether additional legislative changes will be enacted, or whether government regulations, guidance or interpretations will be changed, or what the impact of such changes would be on the marketing approvals, sales, pricing, or reimbursement of our drug candidates or products, if any, may be. We expect that these and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs.
In addition, FDA regulations and guidance may be revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or guidance, or revisions or reinterpretations of existing regulations or guidance, may impose additional costs or lengthen FDA review times for our product candidates. We cannot determine how changes in regulations, statutes, policies, or interpretations when and if issued, enacted, or adopted, may affect our business in the future. Such changes could, among other things, require:
| ● | additional clinical trials to be conducted prior to obtaining approval; |
| ● | changes to manufacturing methods; |
| ● | recalls, replacements, or discontinuance of one or more of our products; and |
| ● | additional recordkeeping. |
Such changes would likely require substantial time and impose significant costs or could reduce the potential commercial value of our product candidates. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any other products would harm our business, financial condition, and results of operations.
Reimbursement rates may vary according to the use of the product and the clinical setting in which it is used, may be based on payments allowed for lower-cost products that are already reimbursed, may be incorporated into existing payments for other products or services, and may reflect budgetary constraints and/or imperfections in Medicare or Medicaid data used to calculate these rates. Net prices for products may be reduced by mandatory discounts or rebates required by government health care programs. Such legislation, or similar regulatory changes or relaxation of laws that restrict imports of products from other countries, could reduce the net price we receive for any future marketed products. As a result, our future products might not ultimately be considered cost-effective. We cannot be certain that reimbursement will be available for any of our product candidates. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, any future products. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize any product candidates that we develop.
| 56 |
Our future growth depends, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend, in part, on our ability to commercialize our product candidates in foreign markets for which we intend to rely on collaborations with third parties. If we commercialize MAT2203 or any other product candidates that we may develop in foreign markets, we would be subject to additional risks and uncertainties, including:
| ● | our customers’ ability to obtain reimbursement for our product candidates in foreign markets; |
| ● | our inability to directly control commercial activities because we are relying on third parties; |
| ● | the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements; |
| ● | different medical practices and customs in foreign countries affecting acceptance in the marketplace; |
| ● | import or export licensing requirements; |
| ● | longer accounts receivable collection times; |
| ● | longer lead times for shipping; |
| ● | language barriers for technical training; |
| ● | reduced protection of intellectual property rights in some foreign countries; |
| ● | foreign currency exchange rate fluctuations; and |
| ● | the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute. |
Foreign sales of our product candidates could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs, any of which may adversely affect our results of operations.
If we market our product candidates in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties.
FDA enforces laws and regulations which require that the promotion of pharmaceutical products be consistent with the approved prescribing information. While physicians may prescribe an approved product for a so-called “off label” use, it is unlawful for a pharmaceutical company to promote its products in a manner that is inconsistent with its approved label and any company which engages in such conduct can subject that company to significant liability. Similarly, industry codes in the EU and other foreign jurisdictions prohibit companies from engaging in off-label promotion and regulatory agencies in various countries enforce violations of the code with civil penalties. While we intend to ensure that our promotional materials are consistent with our label, regulatory agencies may disagree with our assessment and may issue untitled letters, warning letters or may institute other civil or criminal enforcement proceedings. In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include the U.S. Anti-Kickback Statute, U.S. False Claims Act and similar state laws. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
| 57 |
The U.S. Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other. Although there are several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not, in all cases, meet all the criteria for safe harbor protection from anti-kickback liability. Moreover, recent health care reform legislation has strengthened these laws. For example, the ACA, among other things, amends the intent requirement of the U.S. Anti-Kickback Statute and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the U.S. Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the U.S. False Claims Act. Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid.
Over the past few years, several pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicare or Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. Most states also have statutes or regulations similar to the U.S. Anti-Kickback Statute and the U.S. False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include substantial civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, substantial criminal fines and imprisonment.
We have been and expect to be significantly dependent on our collaborative agreements for the development of MAT2203, which exposes us to the risk of reliance on the performance of third parties.
In conducting our research and development activities for MAT2203, we currently rely, and expect to continue to rely, on collaborative agreements with universities, governmental agencies, and not-for-profit organizations for both strategic and financial resources. Key among these agreements is our collaboration agreements with the NIH for the development of MAT2203. The loss of, or failure to perform by us or our partners under any applicable agreements or arrangements, or our failure to secure additional agreements for our product candidates, would substantially disrupt or delay our research and development activities, including our in-process and anticipated clinical trials. Any such loss would likely increase our expenses and materially harm our business, financial condition, and results of operation.
We expect that we will rely on third parties to conduct clinical trials for our product candidates, which exposes us to the risk of reliance on the performance of third parties.
We expect to enter into agreements with third-party CROs, or governmental entities like the NIH, to conduct and manage our clinical programs. We rely heavily on these parties for execution of clinical studies for MAT2203 and our other product candidates and can control only certain and very limited aspects of their activities. Nevertheless, we would be responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and our reliance on the NIH or CROs would not relieve us of our regulatory responsibilities. We, the NIH and our CROs would be required to comply with cGCPs, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for any products in clinical development. The FDA enforces these cGCP regulations through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or the NIH or our CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our clinical trials comply with cGCPs. In addition, our clinical trials must be conducted with products produced under cGMP regulations and will require many test subjects. Our failure or the failure of the NIH or our CROs to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
| 58 |
As a result, many important aspects of our drug development programs would be outside of our direct control. In addition, the NIH or the CROs may not perform all their obligations under their arrangements with us or in compliance with regulatory requirements. If NIH or the CROs do not perform clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, the development and commercialization of MAT2203 or any other product candidates that we may develop may be delayed or our development program may be materially and irreversibly harmed. We cannot control the amount and timing of resources these CROs would devote to our program or our product candidates. If we are unable to rely on the clinical data collected by our CROs, we could be required to repeat, extend the duration of, or increase the size of our clinical trials, which could significantly delay commercialization and require significantly greater expenditures. If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs. As a result of the foregoing, our financial results, and the commercial prospects for MAT2203 and our other product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
We are, and will be, completely dependent on third parties to manufacture our product candidates, and our commercialization of efforts could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of any product candidate or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture the active pharmaceutical ingredient, or API, in MAT2203, or any of our product candidates, for use in our clinical trials or for commercial product, if any. As a result, we will rely on contract manufacturers throughout the development process and then if MAT2203, or any of our product candidates are approved for commercialization. We have not entered into any agreement with any contract manufacturers for commercial supply and may not be able to engage a contract manufacturer for commercial supply of MAT2203, or any of our product candidates, on favorable terms to us, or at all.
The facilities used by our contract manufacturers to manufacture any of our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we submit our NDA to the FDA. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with cGMPs for manufacture of both active drug substances and finished drug products. These cGMP regulations cover all aspects of the manufacturing, testing, quality control and record keeping relating to our product candidates. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of a product candidate or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market such product candidate , if approved.
Our contract manufacturers will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. We do not have control over our contract manufacturers’ compliance with these regulations and standards. Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market any of our product candidates , delays, suspensions or withdrawals of approvals, operating restrictions, and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturers to comply with or maintain any of these standards could adversely affect our ability to develop, obtain regulatory approval for or market any of our product candidates.
| 59 |
If, for any reason, these third parties are unable or unwilling to perform, we may not be able to terminate our agreements with them, and we may not be able to locate alternative manufacturers or formulators or enter into favorable agreements with them and we cannot be certain that any such third parties will have the manufacturing capacity to meet future requirements. If these manufacturers or any alternate manufacturer of finished drug product experiences any significant difficulties in its respective manufacturing processes for our API or finished product or should cease doing business with us, we could experience significant interruptions in product supply or may not be able to create a supply of any product candidate at all. Were we to encounter manufacturing issues, our ability to produce a sufficient product supply might be negatively affected. Our inability to coordinate the efforts of our third-party manufacturing partners, or the lack of capacity available at our third-party manufacturing partners, could impair our ability to supply any product candidate at required levels. Because of the significant regulatory requirements that we would need to satisfy to qualify a new bulk or finished product manufacturer, if we face these or other difficulties with our current manufacturing partners, we could experience significant interruptions in product supply if we decided to transfer manufacturing to one or more alternative manufacturers in an effort to deal with the difficulties.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in lost sales. Additionally, we rely on third parties to supply the raw materials needed to manufacture our potential products. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability, and quality. Any unanticipated disruption to a future contract manufacturer caused by problems at suppliers could delay shipment of our product candidates, increase our cost of goods sold and result in lost sales.
We cannot guarantee that our manufacturing and supply partners will be able to reduce the costs of commercial scale manufacturing of any product candidate over time. If commercial-scale manufacturing costs are higher than expected, these costs may significantly impact our operating results. To reduce costs, we may need to develop and implement process improvements. However, to do so, we will need, from time to time, to notify or make submissions with regulatory authorities, and the improvements may be subject to approval by such regulatory authorities. We cannot be sure that we will receive these necessary approvals or that these approvals will be granted in a timely fashion. We also cannot guarantee that we will be able to enhance and optimize output in our commercial manufacturing process. If we cannot enhance and optimize output, we may not be able to reduce our costs over time.
Outbreaks of communicable diseases may materially and adversely affect our business, financial condition and results of operations.
We face risks related to health epidemics or outbreaks of communicable diseases. Since some of our business partners are outside of the U.S., in China and other Asian countries, including manufacturing operations for our active pharmaceutical ingredient, an outbreak of communicable diseases in Asia or elsewhere, or the perception that such an outbreak could occur, and the measures taken by the governments of countries affected could adversely affect our business, financial condition or results of operations. For example, an outbreak could significantly disrupt our business by limiting our ability to travel or ship materials within or outside China and forcing temporary closure of facilities that we rely upon.
Adverse global conditions, including economic uncertainty, may negatively impact our financial results.
Global conditions, dislocations in the financial markets, or inflation could adversely impact our business. In addition, the global macroeconomic environment has been and may continue to be negatively affected by, among other things, instability in global economic markets, increased U.S. trade tariffs and trade disputes with other countries, instability in the global credit markets, supply chain weaknesses, instability in the geopolitical environment and political tensions, and foreign governmental debt concerns. Such challenges have caused, and may continue to cause, uncertainty and instability in local economies and in global financial markets, which may adversely affect our business.
Risks Relating to Our Intellectual Property Rights and Regulatory Exclusivity
We depend on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from discovering, developing and commercializing product candidates.
We rely upon our LNC Platform and certain of the patents which are exclusively licensed to us by Rutgers. We do not exclusively own some of the patents that underly the LNC Platform. Our rights to use upon the patents we exclusively license are subject to the negotiation of, continuation of and compliance with the terms of our license agreement with Rutgers. Pursuant to the terms of our license agreement with Rutgers, we control the prosecution, maintenance, or filing of the patents to which we hold licenses, as well as the enforcement of these patents against third parties. However, some of our patents and patent applications were either acquired from another company who acquired those patents and patent applications from yet another company or are licensed from a third party. Thus, these patents and patent applications were not written by us or our attorneys, and we did not have control over the drafting and prosecution of certain of these patents. The former patent owners and our licensors might not have given the same attention to the drafting and prosecution of these patents and applications as we would have if we had been the owners of the patents and applications and had control over the drafting and prosecution. We cannot be certain that drafting and/or prosecution of the licensed patents and patent applications by the licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
| 60 |
Our rights to use the technology we license are subject to the validity of the owner’s intellectual property rights. Enforcement of our licensed patents or defense or any claims asserting the invalidity of these patents is often subject to the control or cooperation of our licensors. Legal action could be initiated against the owners of the intellectual property that we license and an adverse outcome in such legal action could harm our business because it might prevent such companies or institutions from continuing to license intellectual property that we may need to operate our business. In addition, such licensors may resolve such litigation in a way that benefits them but adversely affects our ability to use the licensed technology for our products.
Certain of our licenses contained in our agreement with Rutgers contain provisions that allow the licensor to terminate the license if (i) we breach any payment obligation or other material provision under the agreement and fail to cure the breach within a fixed time following written notice of termination, (ii) we or any of our affiliates, licensees or sub licensees directly or indirectly challenge the validity, enforceability, or extension of any of the licensed patents or (iii) we declare bankruptcy or dissolve. Our rights under the licenses are subject to our continued compliance with the terms of the license, including the payment of royalties due under the license. Termination of these licenses may prevent us from discovering, developing, and commercializing product candidates based on the LNC Platform, including our lead anti-infective product candidate MAT2203. Determining the scope of the license and related royalty obligations can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor might attempt to revoke the license. If such an attempt were successful, we might be barred from discovering, developing and commercializing product candidates based on the LNC Platform, including our lead anti-infective product candidates.
It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights.
Our commercial success will depend, in part, on obtaining and maintaining patent protection for our technologies, products and processes, successfully defending these patents against third-party challenges, and successfully enforcing these patents against third party competitors. The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific, and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in interpretations of patent laws may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowable or enforceable in our patents (including patents owned and licensed by us). We currently own or have rights to 30 issued patents relating to our LNC Platform, as well as pending patent applications for our LNC Platform that may never be approved by the United States or foreign patent offices. Furthermore, any patents which may eventually be issued from existing patent applications for any of our technologies, may be challenged, invalidated, or circumvented by third parties and might not protect us against competitors with similar products or technologies.
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights, permit us to gain or keep our competitive advantage, or provide us with any competitive advantage at all. We cannot be certain that any patent application owned by a third party will not have priority over patent applications filed by us, or that we will not be involved in interference, opposition or invalidity proceedings before the United States or foreign patent offices.
| 61 |
We also rely on trade secrets to protect technology, especially in cases where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees, academic collaborators, consultants, and other contractors to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary or licensed information. Typically, research collaborators and scientific advisors have rights to publish data and information in which we may have rights. If we cannot maintain the confidentiality of our proprietary technology and other confidential information, our ability to receive patent protection and our ability to protect valuable information owned by us may be imperiled. Enforcing a claim that a third-party entity illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts are sometimes less willing to protect trade secrets than patents. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
If we fail to obtain or maintain patent or trade secret protection for our technologies, third parties could use our proprietary information, which could impair our ability to compete in the market and adversely affect our ability to generate revenues and attain profitability.
We may also develop trademarks to distinguish our products from the products of our competitors. We cannot guarantee that any trademark applications filed by us or our business partners will be approved. Third parties may also oppose such trademark applications, or otherwise challenge our use of the trademarks. In the event that the trademarks we use are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition, and could require us to devote resources to advertising and marketing new brands. Further, we cannot provide assurance that competitors will not infringe the trademarks we use, or that we will have adequate resources to enforce these trademarks.
Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts.
Our success depends in part on avoiding infringement of the proprietary technologies of others. The pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Identification of third-party patent rights that may be relevant to our proprietary technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Additionally, because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by commercialization of MAT2203 or any future product candidate. There may be certain issued patents and patent applications claiming subject matter that we may be required to license in order to research, develop or commercialize MAT2203 or any future product candidate and we do not know if such patents and patent applications would be available to license on commercially reasonable terms, or at all. Any claims of patent infringement asserted by third parties against us would be time-consuming and may:
| ● | result in costly litigation; |
| ● | divert the time and attention of our technical personnel and management; |
| ● | prevent us from commercializing a product until the asserted patent expires or is held finally invalid or not infringed in a court of law; |
| ● | require us to cease or modify our use of the technology and/or develop non-infringing technology; or |
| ● | require us to enter into royalty or licensing agreements. |
Although no third party has asserted a claim of infringement against us, others may hold proprietary rights that could prevent MAT2203 from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to MAT2203 or our processes could subject us to potential liability for damages and require us to obtain a license to continue to manufacture or market our current product candidates or any future product candidates. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign, MAT2203 or any future product candidates or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing MAT2203 or a future product candidate, which could harm our business, financial condition, and operating results.
| 62 |
We anticipate that competitors may from time to time oppose our efforts to obtain patent protection for new technologies or to submit patented technologies for regulatory approval. Competitors may seek to oppose our patent applications to delay the approval process or to challenge our granted patents, for example, by requesting a reexamination of our patent at the United States Patent and Trademark Office, or the USPTO, or by filing an opposition in a foreign patent office, even if the opposition or challenge has little or no merit. Such proceedings are generally highly technical, expensive and time consuming, and there can be no assurance that such a challenge would not result in the narrowing or complete revocation of any patent of ours that was so challenged.
General Company-Related Risks
We will need to increase the size of our organization to grow our business, and we may experience difficulties in managing this growth.
We have 32 employees as of March 18, 2024. As our development and commercialization plans and strategies develop, we will need to expand the size of our employee base for managerial, development, operational, sales, marketing, financial and other resources. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, motivate, and integrate additional employees. In addition, our management may have to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. Our future financial performance and our ability to commercialize our product candidates and our ability to compete effectively will depend, in part, on our ability to effectively manage any future growth.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. In addition, the loss of the services of certain key employees would adversely impact our business prospects.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. In addition, the loss of the services of certain key employees, including Jerome D. Jabbour, our Chief Executive Officer and President, Theresa Matkovits, our Chief Development Officer, and Dr. Hui Liu, our Chief Technology Officer, would adversely impact our business prospects.
Our ability to compete in the highly competitive pharmaceutical industry depends in large part upon our ability to attract highly qualified managerial, scientific, and medical personnel. To induce valuable employees to remain with us, we intend to provide employees with stock options that vest over time. The value to employees of stock options that vest over time will be significantly affected by movements in our stock price that we will not be able to control and may at any time be insufficient to counteract more lucrative offers from other companies.
Other pharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can develop and commercialize product candidates would be limited.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face a potential risk of product liability because of the clinical testing of MAT2203 or any future product candidates and will face an even greater risk if we commercializeMAT2203 or any other future product. For example, we may be sued if any product we develop or any material that we use in our products allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing, or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of MAT2203. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for MAT2203 or any future products that we may develop; |
| 63 |
| ● | injury to our reputation; |
| ● | withdrawal of clinical trial participants; |
| ● | costs to defend the related litigation; |
| ● | a diversion of management’s time and our resources; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | product recalls, withdrawals or labeling, marketing, or promotional restrictions; |
| ● | loss of revenue; |
| ● | the inability to commercialize our product candidates; and |
| ● | a decline in our stock price. |
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We have obtained product liability insurance covering our clinical trials in the amount of greater than or equal to $5 million in the aggregate. Although we will maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our internal computer systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Despite the implementation of security measures, our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage or disruption from computer viruses, software bugs, unauthorized access, natural disasters, terrorism, war, and telecommunication, equipment and electrical failures. While we have not, to our knowledge, experienced any significant system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our programs. For example, the loss of clinical trial data from completed or ongoing clinical trials for any of our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Moreover, our information security systems and those of our CROs are also subject to laws and regulations requiring that we take measures to protect the privacy and security of certain information gathered and used in our business. For example, HIPAA and its implementing regulations impose, among other requirements, certain regulatory and contractual requirements regarding the privacy and security of personal health information. In the European Union the General Data Protection Regulation, or GDPR, is even more restrictive with respect to all personal information, including information masked by a coding system. In addition to HIPAA and GDPR, numerous other federal and state laws, including, without limitation, state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure, and storage of personal information. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure or theft of confidential or proprietary information, we could incur liability, the further development of our product candidates could be delayed, our competitive position could be compromised, or our business reputation could be harmed.
| 64 |
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We may acquire additional businesses or products, form strategic alliances, or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction.
Risks related to our Securities
Pursuant to the terms of our Series A Preferred Stock, we may be obligated to pay significant royalties.
Pursuant to the terms of the Certificate of Designations of Preferences, Rights and Limitations (the “Certificate of Designations”) for our Series A Preferred Stock, we are required to pay royalties of up to $35 million per year. If and when we obtain FDA or EMA approval of MAT2203 and/or MAT2501, which we do not expect to occur before 2027, if ever, and/or if we generate sales of such products, or we receive any proceeds from the licensing or other disposition of MAT2203 or MAT2501, we are required to pay to certain former holders of our Series A Preferred Stock, in aggregate, a royalty equal to (i) 4.5% of Net Sales (as defined in the Certificate of Designations), subject in all cases to a cap of $25 million per calendar year, and (ii) 7.5% of Licensing Proceeds (as defined in the Certificate of Designations), subject in all cases to a cap of $10 million per calendar year. The Royalty Payment Rights will expire when the patents covering the applicable product expire, which is currently expected to be in 2033.
The rights of the holders of common stock may be impaired by the potential issuance of preferred stock.
Our articles of incorporation give our board of directors the ability to designate and issue preferred stock in one or more series. As a result, the board of directors may, without stockholder approval, issue new series of preferred stock with voting, dividend, conversion, liquidation, or other rights which could adversely affect the relative voting power and equity interest of the holders of common stock. Additional issuances of preferred stock, which could be issued with the right to more than one vote per share, could have the effect of discouraging, delaying, or preventing a change of control of us. The possible impact on takeover attempts could adversely affect the price of our common stock. Although we have no present intention to designate any new series, or issue any shares, of preferred stock, we may do so in the future.
We do not intend to pay dividends on our common stock in the foreseeable future.
The Board of Directors will determine, in its sole discretion, our dividend policy after considering our financial condition, results of operations and capital requirements, as well as other factors. We do not anticipate paying cash dividends on our common stock in the foreseeable future and you should not invest in us with the anticipation of receiving dividend income.
An active public trading market for our common stock may not be sustained.
Although our common stock is listed on the NYSE American, the market for our shares has demonstrated varying levels of trading activity, and we cannot assure you that an active trading market will be sustained. A lack of an active market may impair your ability to sell shares of our common stock at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the price of shares of our common stock. An inactive market may also impair our ability to raise capital by selling shares of capital stock and may impair our ability to acquire other companies or technologies by using our common stock as consideration.
| 65 |
Our share price has been and could remain volatile.
The market price of our common stock has historically experienced and may continue to experience significant volatility. Our progress in developing our product candidates, the impact of government regulations on our products and industry, the potential sale of a large volume of our common stock by stockholders, our quarterly operating results, changes in general conditions in the economy or the financial markets and other developments affecting us or our competitors could cause the market price of our common stock to fluctuate substantially with significant market losses. If our stockholders sell a substantial number of shares of common stock, especially if those sales are made during a short period of time, those sales could adversely affect the market price of our common stock and could impair our ability to raise capital. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies for reasons unrelated to their operating performance and may adversely affect the price of our common stock. In addition, we could be subject to a securities class action litigation as a result of volatility in the price of our stock, which could result in substantial costs and diversion of management’s attention and resources and could harm our stock price, business, prospects, results of operations and financial condition.
If securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. Our research coverage by industry and financial analysts is currently limited. Even if our analyst coverage increases, if one or more of the analysts who cover us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We could be delisted from the NYSE American, which could seriously harm the liquidity of our stock and our ability to raise capital.
We had in the past, and may have in the future, difficulty satisfying NYSE American listing requirements for our common stock. If we are unable to regain or maintain such compliance, we may cease to be eligible to trade on Nasdaq. In such event:
| ● | We may have to pursue trading on a less recognized or accepted market, such as the OTC Bulletin Board or the “pink sheets.” |
| ● | Shares of our common stock could be less liquid and marketable, thereby reducing the ability of stockholders to purchase or sell our shares as quickly and as inexpensively as they have done historically. If our stock is traded as a “penny stock,” transactions in our stock would be more difficult and cumbersome. |
| ● | We may be unable to access capital on favorable terms or at all, as companies trading on alternative markets may be viewed as less attractive investments with higher associated risks, such that existing or prospective institutional investors may be less interested in, or prohibited from, investing in our common stock. This may also cause the market price of our common stock to decline. |
A reverse-stock split of our common stock may not have the intended consequences.
On November 1, 2023, our shareholders approved an amendment to our Certificate of Incorporation to effect a reverse stock split at a ratio in the range of 1-for-2 to 1-for-50, to be determined at the discretion of the Board, and to reduce the total number of authorized shares of common stock from 500,000,000 to 250,000,000, which we refer to as the reverse stock split and authorized share reduction, respectively. We believe that the reverse stock split may result in an increase to the market price of our common stock, enhance the appeal of our common stock to the financial community, improve the trading liquidity of our common stock, make it possible for us to “up-list” our common stock to a higher tier stock exchange, and make us eligible for inclusion on certain biotechnology and pharmaceutical trading indices and exchange-traded funds.
However, we cannot assure you that the reverse stock split, if implemented, will result in any anticipated benefits, including an increase of the market price of our common stock in proportion to the reduction in the number of shares of our common stock outstanding. Moreover, a reverse-stock split may have the effect of decreasing our overall market value. There can be no assurance that the reverse stock split will result in a per share price that will attract institutional investors or investment funds or that such share price will satisfy the investing guidelines of institutional investors or investment funds or improve the trading liquidity of our common stock.
Moreover, the number of shares of our common stock available for issuance following the implementation of the reverse stock split would increase to the extent the reverse stock split reduces the number of outstanding shares of our common stock relative to the number of authorized shares. Such available shares may be used for future corporate purposes, including future acquisitions, investment opportunities, the establishment of collaboration or other strategic agreements, capital raising transactions involving equity or convertible debt securities, future at the market offerings of common stock, or issuance under current or future employee equity plans, and the issuance of equity securities in connection with such transactions may result in potentially significant dilution of our current stockholders’ ownership interests in us.
Upon dissolution of our company, you may not recoup all or any portion of your investment.
In the event of a liquidation, dissolution or winding-up of our company, whether voluntary or involuntary, the proceeds and/or assets of our company remaining after giving effect to such transaction, and the payment of all of our debts and liabilities will be distributed first to the holders of our preferred stock and thereafter to the stockholders of common stock (including the holders of our preferred stock on an “as converted” basis) on a pro rata basis. There can be no assurance that we will have available assets to pay to the holders of common stock, or any amounts, upon such a liquidation, dissolution or winding-up of our Company. In this event, you could lose some or all of your investment.
| 66 |
Our certificate of incorporation allows for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our board of directors has the authority to issue up to 10,000,000 additional shares of our preferred stock without further stockholder approval. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders.
Anti-takeover provisions of our certificate of incorporation, bylaws and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our amended and restated certificate of incorporation and bylaws could discourage, delay, or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your Shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. These provisions also could limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions, among other things:
| ● | they provide that special meetings of stockholders may be called only by the board of directors, President, or our Chairman of the Board of Directors, or at the request in writing by stockholders of record owning at least fifty (50%) percent of the issued and outstanding voting shares of common stock; |
| ● | they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and |
| ● | they allow us to issue, without stockholder approval, up to 10,000,000 shares of preferred stock (all of which remain available for issuance) that could adversely affect the rights and powers of the holders of our common stock. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
Stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees may be limited.
Our certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim for breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine. Our certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act, subject to and contingent upon a final adjudication in the State of Delaware of the enforceability of such exclusive forum provision. These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. For example, stockholders who do bring a claim in the Court of Chancery could face additional litigation costs in pursuing any such claim, particularly if they do not reside in or near the State of Delaware. The Court of Chancery and federal district courts may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments or results may be more favorable to us than to our stockholders. Some companies that adopted a similar federal district court forum selection provision are currently subject to a suit in the Chancery Court of Delaware by stockholders who assert that the provision is not enforceable. If a court were to find either choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition. For example, the Court of Chancery of the State of Delaware recently determined that the exclusive forum provision of federal district courts of the United States of America for resolving any complaint asserting a cause of action arising under the Securities Act is not enforceable. As a result of this decision, we do not currently intend to enforce the federal forum selection provision in our certificate of incorporation, unless the decision is reversed on appeal. However, if the decision is reviewed on appeal and ultimately overturned by the Delaware Supreme Court, we would enforce the federal district court exclusive forum provision.
| 67 |
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As a result of our merger with Aquarius Biotechnologies, Inc., our ability to utilize our U.S. federal net operating loss, carryforwards and U.S. federal tax credits may be limited under Sections 382 of the Internal Revenue Code of 1986, as amended. The limitations apply if an “ownership change,” as defined by Section 382 and Section 383, occurs. Generally, an ownership change occurs if the percentage of the value of the stock that is owned by one or more direct or indirect “five percent shareholders” increases by more than 50 percentage points over their lowest ownership percentage at any time during the applicable testing period (typically three years). In addition, future changes in our stock ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 and Section 383 limitations. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards and other tax attributes to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, the Tax Act, among other things, imposes significant additional limitations on the deductibility of interest and limits net operating loss (NOL) deductions to 80% of net taxable income for losses arising in taxable years beginning after December 31, 2017.
| Item 1B. | Cybersecurity |
Cybersecurity Risk Management and Strategy
We, like other companies in our industry, face several cybersecurity risks in connection with our business. Our business strategy, results of operations, and financial condition have not, to date, been affected by risks from cybersecurity threats. During the reporting period, we have not experienced any material cyber incidents, nor have we experienced a series of immaterial incidents, which would require disclosure.
In the ordinary course of our business, we use, store and process data including data of our employees, trial participants, partners, clients, and vendors. We have implemented a cybersecurity risk management program that is designed to identify, assess, and mitigate risks from cybersecurity threats to this data and our systems. Our cybersecurity risk management program incorporates several components, including information security program assessments, continuous monitoring of cyber risks and threats using automated tools, written incident response and disaster recovery policies and procedures, and employee training. Under the direction of our Chief Financial Officer, our cyber risk management program is led by a third-party IT consultant with more than 30 years of technology engineering experience and several advanced degrees. We also deploy endpoint detection software and device management in conjunction with other reputable cybersecurity software. Additionally, we require multifactor authentication across all systems.
We periodically engage third parties to conduct risk assessments and other vulnerability analyses. Lastly, our program includes cybersecurity training for all employees. The semi-annual training focuses on cyber threat awareness, including phishing.
Governance Related to Cybersecurity Risks
Under the ultimate direction of our chief executive officer (“CEO”), the Board of Directors is updated on our cybersecurity risk management program, including any critical cybersecurity risks, ongoing cybersecurity initiatives and strategies, and applicable regulatory requirements and industry standards, on an as-needed basis. The CEO also notifies the Board of any cybersecurity incidents (suspected or actual) and provides updates on the incidents as well as cybersecurity risk mitigation activities as appropriate.
| 68 |
| Item 2. | Properties |
Facilities
Our administrative offices consist of approximately 8,900 square feet of office space in Bedminster, NJ that we occupy under a lease that expires in June 2029. We also lease laboratory space approximating 14,000 square feet in Bridgewater, NJ, that expires in July 2027.
| Item 3. | Legal Proceedings |
We are not currently a party to any legal proceedings, and we are not aware of any claims or actions pending or threatened against us. In the future, we might from time to time become involved in litigation relating to claims arising from our ordinary course of business.
| Item 4. | Mine Safety Disclosures |
Not applicable
PART II
| Item 5. | Market For Registrant’s Common Equity, Related Stockholder Matters And Issuer Purchases Of Equity Securities |
Our common stock is quoted on the NYSE American under the symbol “MTNB”.
On March 18, 2024, the closing sale price of our common stock, as reported by the NYSE MKT, was $0.27 per share and we had approximately 102 record holders of our common stock. The number of record holders was determined from the records of our transfer agent and does not include beneficial owners of common stock whose shares are held in the names of various security brokers, dealers, and registered clearing agencies. VStock Transfer, LLC is the transfer agent and registrar for our common stock.
Dividends
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
Recent Sales of Unregistered Securities
None.
Repurchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not purchase any of our registered securities during the period covered by this Annual Report.
| Item 6. | [Reserved] |
| 69 |
| Item 7. | Management’s Discussion And Analysis Of Financial Condition And Results Of Operations |
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and financing needs, includes forward-looking statements that involve risks and uncertainties and should be read together with the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those discussed below and elsewhere in this Annual Report and in other reports we file with the Securities and Exchange Commission, particularly those under “Risk Factors.” All dollars amounts stated in the tabular and paragraph formats are presented in thousands, except per share data, or otherwise indicated.
Overview
We are a clinical-stage biopharmaceutical company focused on delivering groundbreaking therapies using our lipid nanocrystal (LNC) platform delivery technology (LNC Platform). We are seeking to develop an internal pipeline of products utilizing the LNC Platform to successfully encapsulate small molecules and small oligonucleotides and facilitate targeted and extrahepatic delivery to desired cells tissues without toxicity.
Key elements of our strategy include:
| ● | Advancing MAT2203 into the ORALTO trial for the treatment of invasive aspergillosis in patients with limited treatment options by securing a development and/or commercial partner. This initial indication is designed to be a gateway indication to establish the pharmacodynamic bridge necessary to expand the use of MAT2203 into other indications to treat deadly invasive fungal infections (e.g., mucormycosis, candidiasis and the endemic mycoses) through limited additional clinical work under a 505(b)(2) pathway, thereby making MAT2203 a pipeline in a product. | |
| ● | Expanding the utilization of our LNC Platform with other small molecules and small oligonucleotides into inflammation and oncology in order to develop an internal pipeline of differentiated drug candidates. Oral, extrahepatic and non-toxic intracellular delivery of these molecules would represent a significant advancement. | |
| ● | Building an external pipeline of collaborations focused on our LNC Platform with leading pharmaceutical companies to provide delivery solutions for their complex small molecules and small oligonucleotides, including ASOs and siRNAs. |
For the years ended December 31, 2023 and 2022, our net loss was $22,942 and $20,997, respectively. We have incurred losses for each period from our inception and expect to incur additional losses for the foreseeable future. We do not believe that the cash, cash equivalents and marketable debt securities on hand are sufficient to fund planned operations beyond the next twelve months from the filing date of this Annual Report. We will seek to fund our operations through public or private equity offerings, debt financings, government or other third-party funding, collaborations, and licensing arrangements. These financing alternatives may not be available to us on acceptable terms, or at all. As a result, substantial doubt exists about our ability to continue as a going concern.
Financial Operations Overview
Revenue
During the year ended December 31, 2023, we generated $1,096 in contract research revenue resulting from the research collaboration with BioNTech SE and Genentech Inc. During the year ended December 31, 2022, we generated $3,188 in contract revenue resulting from the research collaboration with BioNTech SE. Our ability to generate product revenue, which we do not expect to occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of our early-stage product candidates.
| 70 |
Research and Development Expenses
Research and development expenses consist of costs incurred for the development of product candidate MAT2203, and advancement of our LNC Platform, which include:
| ● | the cost of acquiring, developing, and manufacturing pre-clinical and human clinical trial materials; |
| ● | costs for consultants and contractors associated with Chemistry and Manufacturing Controls (CMC), pre-clinical and clinical activities and regulatory operations; |
| ● | expenses incurred under agreements with contract research organizations, or CROs, including the NIH, that conduct our pre-clinical or clinical trials; |
| ● | employee-related expenses, including salaries and stock-based compensation expense for those employees involved in the research and development process; and |
| ● | the reimbursement of certain expenses related to the CFF award agreement. |
The table below summarizes our direct research and development expenses for our product candidates and development platform for the years ended December 31, 2023 and 2022. Our direct research and development expenses consist principally of external costs, such as fees paid to contractors, consultants, analytical laboratories and CROs and/or the NIH, in connection with our development work. We typically use our employee and infrastructure resources for manufacturing clinical trial materials, conducting product analysis, study protocol development and overseeing outside vendors. Included in “Internal Staffing, Overhead and Other” below is the cost of laboratory space, supplies, research and development (R&D) employee costs (including stock option expenses), travel and medical education.
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Direct research and development expenses: | ||||||||
| Manufacturing process development | $ | 1,182 | $ | 2,523 | ||||
| Preclinical trials | 852 | 720 | ||||||
| Clinical development | 1,696 | 2,175 | ||||||
| Regulatory | 581 | 737 | ||||||
| Internal staffing, overhead and other | 10,178 | 10,523 | ||||||
| Total research & development | $ | 14,489 | $ | 16,678 | ||||
Research and development activities are central to our business model. We expect our research and development expenses to increase over time because product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage human trials. However, we anticipate that our research and development expenses during 2024 will be lower compared with expenses incurred during 2023 until such time as we are able to secure additional funding to support initiation of our Phase 3 registration trial for MAT2203 and advancement of our LNC platform delivery technology.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive and finance functions. Other general and administrative expenses include facility costs, insurance, investor relations expenses, professional fees for legal, patent review, consulting, and accounting/audit services. We anticipate that our general and administrative expenses during 2024 will decrease slightly compared to expenses incurred during 2023.
| 71 |
Sale of Net Operating Losses (NOLs) & Tax Credits
Income obtained from selling unused net operating losses (NOLs) and research and development tax credits under the New Jersey Technology Business Tax Certificate Program was $484 and $3,491 for the years ended December 31, 2023 and 2022, respectively. The income recorded in 2023 included sales related to tax year 2022, while income recorded in 2022 included sales related to tax years 2021 and 2020.
Other Income, net
Other income, net is largely comprised of interest income (expense) and dividends.
Application of Critical Accounting Policies and Accounting Estimates
A critical accounting policy is one that is both important to the portrayal of our financial condition and results of operation and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain.
For a description of our significant accounting policies, refer to “Note 3 – Summary of Significant Accounting Policies.” Of these policies, the following are considered critical to an understanding of our Consolidated Financial Statements as they require the application of the most difficult, subjective and complex judgments; (i) Research and development costs, and (ii) Goodwill and other intangible assets.
Current Operating Trends
Our current R&D efforts are focused on advancing our lead LNC product candidate, MAT2203, through clinical development and expanding application of our LNC Platform through both internal efforts and collaborations with third parties. Our R&D expenses consist of manufacturing work and the cost of active pharmaceutical ingredients and excipients used in such work, fees paid to consultants for work related to clinical trial design and regulatory activities, fees paid to providers for conducting various clinical studies as well as for the analysis of the results of such studies, and for other medical research addressing the potential efficacy and safety of our drugs. We believe that significant investment in product development is a competitive necessity, and we plan to continue these investments to be in a position to realize the potential of our product candidates and proprietary technologies.
We expect that most of our R&D expenses in the near-term future will be incurred in support of our current and future preclinical and clinical development programs. These expenditures are subject to numerous uncertainties relating to timing and cost to completion. We test compounds in numerous preclinical studies for safety, toxicology, and efficacy. At the appropriate time, subject to the approval of regulatory authorities, we expect to conduct early-stage clinical trials. We anticipate funding these trials ourselves, and possibly with the assistance of federal grants, contracts, or other agreements. As we obtain results from trials, we may elect to discontinue or delay clinical trials for certain products to focus our resources on more promising products. Completion of clinical trials may take several years, and the length of time varies substantially according to the type, complexity, novelty and intended use of a product candidate.
The commencement and completion of clinical trials for our products may be delayed by many factors, including lack of efficacy during clinical trials, unforeseen safety issues, slower than expected participant recruitment, lack of funding or government delays. In addition, we may encounter regulatory delays or rejections as a result of many factors, including results that do not support the intended safety or efficacy of our product candidates, perceived defects in the design of clinical trials and changes in regulatory policy during the period of product development. As a result of these risks and uncertainties, we are unable to accurately estimate the specific timing and costs of our clinical development programs or the timing of material cash inflows, if any, from our product candidates. Our business, financial condition and results of operations may be materially adversely affected by any delays in, or termination of, our clinical trials or a determination by the FDA that the results of our trials are inadequate to justify regulatory approval, insofar as cash in-flows from the relevant drug or program would be delayed or would not occur.
| 72 |
Results of Operations
Years Ended December 31, 2023 and 2022
The following table summarizes our operating expenses for the years ended December 31, 2023 and 2022:
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenues | $ | 1,096 | $ | 3,188 | ||||
| Expenses: | ||||||||
| Research and development | $ | 14,489 | $ | 16,678 | ||||
| General and administrative | 10,373 | 11,100 | ||||||
| Operating Expenses | $ | 24,862 | $ | 27,778 | ||||
| Sale of net operating losses (NOLs) | $ | 484 | $ | 3,491 | ||||
Revenues. We generated $1,096 and $3,188 for the years ended December 31, 2023 and 2022, respectively. The amount earned during 2023 consists of contract research revenue resulting from the research collaboration with BioNTech SE and the feasibility study agreement with Genentech Inc., while the amount earned during 2022 resulted exclusively from the research collaboration with BioNTech SE.
Research and Development expenses. R&D expense for the years ended December 31, 2023 and 2022 was $14,489 and $16,678, respectively. The decrease of $2,189 was due to a decrease of $1,302 of clinical trial expenses and $541 decrease in professional & consulting fees, primarily related to the pause of our MAT2203 development program, and a $291 decrease due to the license agreement amendment expense recorded in 2022.
General and Administrative expenses. G&A expense for the years ended December 31, 2023 and 2022 was $10,373 and $11,100, respectively. The decrease of $727 over the prior year was primarily due to a decrease in certain insurance premiums, and lower consulting fees and facility expenses related to certain projects that began in the prior year.
Sale of net operating losses (NOLs) & tax credits. The Company recognized $484 and $3,491 for the years ended December 31, 2023 and 2022, respectively, in connection with the sale of state net operating losses and state research and development credits to a third party under the New Jersey Technology Business Tax Certificate Program. The income recorded in 2023 included sales related to tax year 2022, while income recorded in 2022 included sales related to tax years 2021 and 2020.
Liquidity and capital resources
Sources of Liquidity
We have funded our operations since inception primarily through private placements of our preferred stock and our common stock and common stock warrants. As of December 31, 2023, we have raised a total of $156,851 in gross proceeds and $144,141, net proceeds, from sales of our equity securities since inception in 2013.
As of December 31, 2023, we had cash, cash equivalents and marketable debt securities, excluding restricted cash, totaling $13,756.
2020 At-The-Market Sales Agreement
On July 2, 2020, we entered into an At-The-Market Sales Agreement (the “Sales Agreement”) with BTIG, LLC (“BTIG”), pursuant to which we may offer and sell, from time to time, through BTIG, as sales agent and/or principal, shares of our common stock having an aggregate offering price of up to $50 million, subject to certain limitations on the amount of common stock that may be offered and sold by us set forth in the Sales Agreement. BTIG will be paid a 3% commission on the gross proceeds from each sale. We may terminate the Sales Agreement at any time; BTIG may terminate the Sales Agreement in certain limited circumstances. We did not sell any shares of our common stock under the Sales Agreement during either 2023 or 2022. At December 31, 2023, the Sales Agreement’s available capacity was $44,247.
| 73 |
Cash Flows
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
| Years Ended December 31 | ||||||||
| 2023 | 2022 | |||||||
| Cash used in operating activities | $ | (15,278 | ) | $ | (19,156 | ) | ||
| Cash provided by investing activities | 13,242 | 4,877 | ||||||
| Cash (used in) provided by financing activities | (7 | ) | 79 | |||||
| Net decrease in cash and cash equivalents and restricted cash | $ | (2,043 | ) | $ | (14,200 | ) | ||
Operating Activities
Net cash used in operating activities for the year ended December 31, 2023 was $15,278, compared to $19,156 in the prior year. The decrease of $3,878 for the period was primarily due to a decrease of $6,456 of working capital adjustments due to the timing of receipts and payments in the ordinary course of business, partially offset by a $1,945 increase in net loss in the current period, $280 decrease in stock based compensation expense, and a decrease associated with the license agreement charge of $291 in 2022. We expect the amount of cash used in operations during 2024 to be slightly higher as we continue to move our product candidates and LNC Platform forward in their development cycles.
Investing Activities
Net cash provided by investing activities for the year ended December 31, 2023 was $13,242, compared to $4,877 of net cash provided by investing activities for the year ended December 31, 2022. The increase of $8,365 provided by investing activities was primarily due to a $7,691 net decrease in the purchases and maturities of marketable debt securities and $674 decrease in purchases of equipment and leasehold improvements as compared to the prior year.
Financing Activities
Net cash (used in) and provided by financing activities was ($7) and $79 for the years ended December 31, 2023 and 2022, respectively. The decrease of $86 in cash provided by financing activities is primarily due to the decrease in the receipt of proceeds of $99 from the exercise of stock options in 2022.
Funding Requirements and Other Liquidity Matters
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| ● | conduct further clinical studies of MAT2203, our lead product candidate, even is such studies are primarily financed with non-dilutive funding from NIH; |
| ● | seek to discover and develop additional product candidates; |
| ● | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| ● | require the manufacture of larger quantities of product candidates for clinical development and potentially commercialization; |
| ● | maintain, expand, and protect our intellectual property portfolio; |
| ● | hire additional clinical, quality control and scientific personnel; and |
| 74 |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts and personnel and infrastructure necessary to help us comply with our obligations as a public company. |
We do not believe that our existing cash, cash equivalents and marketable debt securities will be sufficient to fund our operating expenses and capital expenditures requirements beyond the next twelve months from the filing date of this Annual Report. As a result, substantial doubt exists about the Company’s ability to continue as a going concern.
Until such time, if ever, that we can generate product revenues sufficient to achieve profitability, we expect to finance our cash needs through a combination of private and public equity offerings, debt financings, government or other third-party funding, collaborations, and licensing arrangements. To the extent that we raise additional capital through the sale of common stock, convertible securities or other equity securities, the ownership interest of our stockholders may be materially diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights of our common stockholders. Debt financing and preferred equity financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends, that could adversely impact our ability to conduct our business. Securing additional financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development of our product candidates.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Our financial condition and results of operations may also be impacted by other factors we may not be able to control, such as global supply chain disruptions, global trade disputes and/or political instability. Increases in interest rates, especially if coupled with reduced government spending and volatility in financial markets, may have the effect of further increasing economic uncertainty and heightening these risks. Additionally, rising inflation rates may affect us by increasing operating expenses, such as employee-related costs and clinical trial expenses, negatively impacting our results of operations.
Contractual Obligations and Commitments
Refer to Note 10 – “Commitments” in the accompanying notes to the consolidated financial statements for a discussion of the Company’s contractual obligations and commitments.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our balance sheets.
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to Note 3 - “Summary of Significant Accounting Policies,” in the accompanying notes to the consolidated financial statements for a discussion of recent accounting pronouncements.
| Item 7A. | Quantitative And Qualitative Disclosures About Market Risk |
Not applicable.
| 75 |
| Item 8. | Financial Statements And Supplementary Data |
Our
financial statements, together with the independent registered public accounting firm report thereon, are incorporated by reference from
the applicable information set forth in Part IV Item 15, “Exhibits, Financial Statement Schedules” of this Annual Report
on Form 10-K which includes the report of EisnerAmper LLP (PCAOB ID:
| Item 9. | Changes In And Disagreements With Accountants On Accounting And Financial Disclosure |
Not applicable.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Disclosure Controls and Procedures:
As of December 31, 2023, under the supervision and with the participation of our principal executive officer and principal financial officer we have evaluated, the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2023.
Our disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed in the reports that we filed or submitted under the Exchange Act is recorded, processed, summarized and reported within time periods specified by the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to our management, including principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting:
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed by, or under the supervision of, our principal executive officer and principal financial officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. Our control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the consolidated financial statements.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, any projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting based on criteria established in “Internal Control—Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, management has concluded as of December 31, 2023, our internal control over financial reporting was effective, as management did not identify any deficiencies in internal control over financial reporting that was determined to be a material weakness.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
| 76 |
Changes in Internal Control Over Financial Reporting:
There was no change in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that occurred during the quarter ended December 31, 2023 covered by this report that has materially affected or is reasonably likely to materially affect our internal control over financial reporting.
| Item 9B. | Other Information |
None.
| Item 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
None
PART III
| Item 10. | Directors, Executive Officers And Corporate Governance |
All directors hold office for one-year terms until the election and qualification of their successors. Officers are appointed by our board of directors and serve at the discretion of the board, subject to applicable employment agreements. The following table sets forth information regarding our executive officers and the members of our board of directors.
| Name | Age | Position(s) | ||
| Eric Ende | 55 | Chairman of the Board, Director | ||
| Jerome D. Jabbour | 49 | Chief Executive Officer and President, Director | ||
| James J. Ferguson | 70 | Chief Medical Officer | ||
| Thomas J. Hoover | 54 | Chief Business Officer | ||
| Keith A. Kucinski | 54 | Chief Financial Officer | ||
| Hui Liu | 56 | Chief Technology Officer | ||
| Theresa Matkovits | 56 | Chief Development Officer | ||
| Herbert Conrad | 91 | Director | ||
| Kathryn Corzo | 63 | Director | ||
| Natasha Giordano | 63 | Director | ||
| James S. Scibetta | 59 | Director | ||
| Matthew Wikler | 74 | Director |
Management
Jerome D. Jabbour, JD was appointed Chief Executive Officer in March 2018. He has served as our President since March 2016. Prior to that he served as our Executive Vice President, Chief Business Officer, General Counsel and Secretary since October 2013 and as one of our directors from April 2012 until November 2013. Mr. Jabbour is also a Co-founder of Matinas BioPharma. Prior to joining our management team, he was the Executive Vice President and General Counsel of MediMedia USA, or MediMedia, from 2012 to October 2013, a privately held diversified healthcare services company. Prior to MediMedia, he was the Senior Vice President, Head of Global Legal Affairs of Wockhardt Limited (2008-2012), a global pharmaceutical and biotechnology company, and Senior Counsel and Assistant Secretary at Reliant (2004-2008). Earlier in his career, he held positions as Commercial Counsel at Alpharma, Inc. (2003-2004) and as a Corporate Associate at Lowenstein Sandler LLP (1999-2003). Mr. Jabbour earned his J.D. from Seton Hall University School of Law in New Jersey and a B.A. in Psychology from Loyola University in Baltimore.
| 77 |
James J. Ferguson, MD was appointed Chief Medical Officer in February 2019. Prior to joining the Company, he served as the Cardiovascular and Bone Therapeutic Area Head for U.S. Medical Affairs, at Amgen (NASDAQ: AMGN), multinational biopharmaceutical company, from 2016 to 2019. Prior to Amgen Dr. Ferguson held a number of senior positions at AstraZeneca, a multinational pharmaceutical and biopharmaceutical company, including Vice President of U.S. Cardiovascular Medical and Scientific External Relations, Therapeutic Area Vice President of Cardiovascular Global Medical Affairs, U.S. Development Brand Leader for BRILINTA®, and Senior Director, Clinical Research. Before joining AstraZeneca he was Vice President of Surgical and Critical Care for The Medicines Company. In addition, Dr. Ferguson had more than 20 years of academic experience as the Associate Director of Clinical Cardiology Research at the Texas Heart Institute, Co-Director of the Cardiology Fellowship Training Program at St. Luke’s Episcopal Hospital in Houston, where he was an Associate Professor of Medicine at Baylor College of Medicine, and a Clinical Assistant Professor at the University of Texas Health Science Center at Houston. Dr. Ferguson has served on the Editorial Board of numerous peer-reviewed journals and has over 400 publications and book chapters. Dr. Ferguson received his B.A. (cum Laude) in Biology from Harvard University, his M.D. from the University of Pennsylvania School of Medicine and completed his post-graduate training at the University of Michigan Medical Center, Ann Arbor, Michigan and Beth Israel Hospital, Boston, Massachusetts.
Thomas J. Hoover, MBA has served as Chief Business Officer since December 2021. Prior to joining the Company, Mr. Hoover was the Chief Business Officer at Millendo Therapeutics (now Tempest Therapeutics (NASDAQ: TPST)), a public, clinical stage biotech, from 2016 to 2021. Prior to joining Millendo, Mr. Hoover was Vice President of New Product Planning and Corporate Development and Licensing at Sunovion Pharmaceuticals Inc, a global biopharmaceutical company. Mr. Hoover started his pharmaceutical career at GSK in 2001 working in the Global Commercial Strategy group. Earlier in his career, Mr Hoover worked for The Boston Consulting Group. Mr. Hoover holds an M.B.A. from the University of North Carolina and a B.A. from Harvard College.
Keith A. Kucinski, MBA, CPA was appointed Chief Financial Officer in January 2019. He most recently served as Chief Financial Officer at RemedyOne, a privately held healthcare consulting organization, during 2018. Prior to that, he served as Vice President & Treasurer at Par Pharmaceutical Companies, Inc., an operating company of Endo International plc, a leading generics and specialty-branded pharmaceutical company, from 2009 to 2015. In addition, Mr. Kucinski held various roles at Barr Pharmaceuticals, Inc., including Senior Director, Finance & Corporate Development and Assistant Treasurer & Senior Director, Finance. Mr. Kucinski is a Certified Public Accountant. He received his Bachelor of Business Administration in Accounting from the University of Notre Dame and an M.B.A. in Finance & Management from the Leonard N. Stern School of Business at New York University.
Hui Liu, PhD, MBA has serves as Chief Technology Officer since December 2020. Prior to joining the Company, Dr. Liu was Director of Formulation and Delivery at Seqirus USA Inc., a privately held global leader in influenza and pandemic response, from 2017 to 2020. Prior to joining Seqirus, Dr. Liu was Director of CMC at Cellics Therapeutics, Inc., a privately held development stage biopharmaceutical company, in 2017, and Senior Technical Lead at Alcon Inc. (SIX/NYSE: ALC), a global leader in eye care, from 2015 to 2017. Earlier in his career, Dr. Liu held positions at Cellics Therapeutics, Inc., a privately held biotech company, and Allergan. Dr. Liu holds a Ph.D. in polymer chemistry from the University of Michigan, an M.B.A. from the University of Massachusetts, Amherst, and a B.S. from The University of Science and Technology of China.
Theresa Matkovits, PhD has served as Chief Development officer since October 2018. She joined the Company after having most recently served as the Chief Operating Officer of ContraVir Pharmaceuticals (NASDAQ: CTRV) (now Hepion Pharmaceuticals), a clinical stage biopharmaceutical company, from 2015 to 2018. From 2013 to 2015, Dr. Matkovits served as Global Program Leader at NPS Pharmaceuticals, a specialty pharmaceutical company that was purchased by Shire in 2015. Prior to her time at NPS, Dr. Matkovits was Vice President, Innovation Leader at The Medicines Company. Earlier in her career, Dr. Matkovits held a number of global leadership positions at Novartis across Global Development and the U.S. Commercial Organization, including as Head, Strategic Planning and Operations, U.S. Medical and Drug Regulatory Affairs. Dr. Matkovits began her career at the Roche Institute of Molecular Biology and Organon where she held positions in clinical development in women’s health and research in the area of infertility. Dr. Matkovits serves on the Board of Directors of Appili Therapeutics (TSX: APLI; OTCQX: APLIF), and is Chairperson of GoodCap Pharmaceuticals, a privately held pharmaceutical company. Dr. Matkovits earned her Ph.D. in Biochemistry and Molecular Biology from the University of Medicine and Dentistry of NJ.
| 78 |
Directors
Eric Ende has served on our board of directors since April 2017 and was selected to be Chairman of the Board of Directors of our Company in October, 2022. Dr. Ende is president of Ende BioMedical Consulting Group, a privately-held consulting company which is focused on helping life sciences companies raise capital, identify licensing partners, and optimize corporate structure as well as analyzing both private and public investment opportunities for clients within the life sciences industry, a position he has held since 2009. In addition, Dr. Ende consulted with Icahn Enterprises in their efforts to appoint board members at Forest Labs, Genzyme, Biogen IDEC, and Amylin. Dr. Ende served on the board of directors and as a member of the Audit and Risk Management Committee of Genzyme Corp. (NASDAQ: GENZ) from 2010 until it was acquired by Sanofi (NSYE: SNY) in 2011. Through another activist campaign, Dr. Ende served on the board of directors of Progenics Pharmaceuticals, Inc., an oncology company, from 2019 until it was acquired by Lantheus Holdings, Inc. in 2020, as Chair of the Compensation Committee and a member of the Audit and Science Committees. Dr. Ende also serves on the board of directors of Avadel Pharmaceuticals plc, a biopharmaceutical company, as Chair of the Nomination & Corporate Governance Committee and a member of the Audit and Compensation Committees. Dr. Ende is currently serving on the Technology Transfer Committee of Mount Sinai Innovation Partners and served as the Chairman of the Unsecured Creditor’s Committee overseeing the bankruptcy of Egenix, Inc. From 2002 through 2008, Dr. Ende was the senior biotechnology analyst at Merrill Lynch. From 2000 through 2002, Dr. Ende was the senior biotechnology analyst at Banc of America Securities and, from 1997 to 2000, he was a biotechnology analyst at Lehman Brothers. Dr. Ende received an MBA in Finance & Accounting from NYU – Stern Business School in 1997, an MD from Mount Sinai School of Medicine in 1994, and a BS in Biology and Psychology from Emory University in 1990. We believe Dr. Ende is qualified to serve on our board of directors due to his industry experience, including as president of Ende BioMedical Consulting Group and as a biotechnology analyst, and his prior public company board experience.
Jerome D. Jabbour. See description under “Management.”
Herbert Conrad served as our Chairman of the Board from July 2013 until October 2022, and as Chairman of the Board of Matinas BioPharma, Inc. from October 2012 until October 2022. He continues to serve as a member of our Board of Directors today. He also serves on the board of directors of Celldex Therapeutics, Inc. (NASDAQ: CLDX), biopharmaceutical company focused on the development and commercialization of immunotherapies and other targeted biologics, and as an Advisor to the Seaver Autism Center at Mount Sinai Hospital. Mr. Conrad was the President of the U.S. Pharmaceuticals Division of Hoffmann-La Roche, Inc. from 1982 until his retirement in 1993. Prior to that, he held many positions of increasing responsibility at Roche Pharmaceuticals in the United States. Mr. Conrad previously served on the board of directors of Arbutus Biopharma Corporation (NASDAQ: ABUS), Pharmasset, Inc. (chairman), Savient Pharmaceuticals, Inc. (NASDAQ: SVNT), Dura Pharmaceuticals, Inc., UroCor, Inc., GenVec, Inc. (NASDAQ: GNVC) (chairman), Sicor, Inc., Bone Care International, Inc. (chairman), Sapphire Therapeutics, Inc. (chairman), the medical advisory board of Henry Schein Inc. (NASDAQ: HSIC), and he was a Director and Co-Founder of Reliant Pharmaceuticals. Pharmasset was acquired by Gilead Sciences, Inc. for $11 billion in 2011 and Reliant was acquired by GlaxoSmithKline for $1.65 billion in 2007. He received B.S. and M.S. degrees from the Brooklyn College of Pharmacy and an honorary Doctorate in Humane Letters from Long Island University. We believe Mr. Conrad is qualified to serve on our board of directors due to his extensive expertise and experience in the life sciences industry and his extensive board experience.
Kathryn Corzo has served on our board of directors since September 2021 and is currently the Chief Operating Officer of bit.bio. Prior to bit.bio, Ms. Corzo was a partner at Takeda Ventures, Inc., the corporate investment arm of Takeda Pharmaceutical Company Limited, and previously Head, Oncology Cell Therapy Development at Takeda Pharmaceutical Company Limited (TSE: 4502/NYSE: TAK), a global biopharmaceutical company, a position she has held since 2020. Prior to joining Takeda, Ms. Corzo held positions of increasing responsibility at Sanofi Genzyme, a specialty care global business unit of Sanofi, from 2010 to 2019. Prior to joining Sanofi, Ms. Corzo worked at Hoffman – La Roche, Roche Molecular Systems, Eli Lilly and Syndax from 1989 to 2010, during which time she held roles of increasing seniority in operations, global clinical development, medical affairs, business development, market access and brand management across multiple therapeutic products and indications. We believe Ms. Corzo is qualified to serve on our Board of Directors due to her broad experience in the life sciences industry.
Natasha Giordano. Ms. Giordano has served as a member of our board of directors since September 2020. Ms. Giordano has been President, Chief Executive Officer and director of PLx Pharma Inc. (NASDAQ: PLXP) since January 2016. Previously, Ms. Giordano served as Chief Executive Officer of ClearPoint Learning, Inc., a privately held learning and training platform company, from May 2015 through November 2015. She also served on the ClearPoint board of directors from December 2009 through November 2015. Previously, Ms. Giordano served as the Chief Executive Officer of Healthcare Corporation of America (NYSE: HCA), a leading healthcare provider, from January 2014 through August 2014. From June 2009 to August 2012, Ms. Giordano served as Chief Operating Officer and then as Chief Executive Officer, President and a member of the board of directors of Xanodyne Pharmaceuticals, Inc., a privately-held a branded specialty pharmaceutical company with development and commercial capabilities focused on pain management and women’s health. Prior to that, she served as President, Americas, for Cegedim Dendrite (formerly Dendrite International Inc.), a global technology services company, from 2007 to 2008 and as Senior Vice President of the Global Customer Business Unit of Cegedim Dendrite from 2004 to 2007. Ms. Giordano holds a Bachelor of Science degree in nursing from Wagner College. We believe Ms. Giordano is qualified to serve as a director due to her extensive experience in commercialization, general management and knowledge of the pharmaceutical and health care industries.
| 79 |
James S. Scibetta has served as a member of our board of directors since November 2013. Mr. Scibetta is currently a senior consultant with Rock Springs Capital, an investment firm that manages long-biased, healthcare-focused public equity funds. Between 2021 and 2023, Mr. Scibetta was the Chief Executive Officer of ImmuneID, Inc., a privately held company that leverages existing antibody responses to rapidly unveil the complexities of the immune system, thereby revealing pathways leading to precise, transformative therapies. Prior to ImmuneID, Mr. Scibetta was Chief Executive Officer of Maverick Therapeutics, a development stage immune-oncology company, from 2017 until 2021 when it was acquired by Takeda Pharmaceutical Company Limited. Prior to Maverick, he was President and Chief Financial Officer of Pacira Pharmaceuticals, Inc. (NASDAQ: PCRX), a specialty pharmaceutical company, a position he has held since October 2015. Prior to that, Mr. Scibetta was the Chief Financial Officer of Pacira since 2008. Prior to joining Pacira in August 2008, he served as a consultant to Genzyme Corporation following the sale of Bioenvision Inc. (NASDAQ: BIVN) to Genzyme in 2007. From 2006 to 2007 Mr. Scibetta was CFO of Bioenvision. From 2001 to 2006, he was Executive Vice President and Chief Financial Officer of Merrimack Pharmaceuticals Inc. (NASDAQ: MACK). Mr. Scibetta has previously served on the board of directors at the following life sciences companies: Nephros Inc. (NASDAQ: NEPH), Merrimack Pharmaceuticals and Labopharm Inc. Prior to his executive management experience, Mr. Scibetta spent over a decade in investment banking where he was responsible for sourcing and executing transactions for a broad base of public and private healthcare and life sciences companies. Mr. Scibetta received his Bachelor of Science in Physics from Wake Forest University and an MBA from the University of Michigan. We believe Mr. Scibetta is qualified to serve on our board of directors because of his extensive management experience in the pharmaceutical industry, his investment banking experience and his experience as a chief financial officer and audit committee member of several publicly traded companies.
Matthew A. Wikler has served as a member of our board of directors since January 2018. Dr. Wikler currently serves as the Principal of Infectious Disease Technology Development Consulting (IDTD Consulting), a privately-held consulting firm, where he provides clinical, medical and regulatory strategic insight to companies developing new technologies for the treatment and prevention of infectious diseases, a position he has held since 2015. Prior to that from 2012 to 2015, Dr. Wikler served at The Medicines Company (NASDAQ: MDCO), a biopharmaceutical company, as VP, New Business Ventures and VP and Medical Director, Infectious Disease Care. Over the course of his career Dr. Wikler held senior leaderships positions for a number of pharmaceutical companies, including as Chief Development Officer of Rib-X Pharmaceuticals, Inc., a privately-held biopharmaceutical company developing new antibiotics to provide expanded coverage, safety and convenience for the treatment of serious and life-threatening infections, President and Chief Executive Officer of IASO Pharma Inc., a privately-held clinical stage biotechnology company focused on the development of antibacterial and antifungal therapeutics, the Institute for One World Health, a 501(c)(3) nonprofit drug development organization, Mpex Pharmaceuticals, Inc., a privately-held company focused on developing and manufacturing therapies for antibiotic resistance with focus on gram-negative organisms, Peninsula Pharmaceuticals, Inc., a privately held biopharmaceutical company focused on developing and commercializing antibiotics to treat life-threatening infections (acquired by Johnson & Johnson (NYSE: JNJ)), ViroPharma Incorporated (NASDAQ: VPHM), Bristol-Myers Squibb Company (NYSE:BMY), and Ortho-McNeil Pharmaceutical (a division of Johnson & Johnson). Dr. Wikler began his career at Smith Kline & French/Smith Kline Beecham where he held positions of increasing responsibilities over ten years. Dr. Wikler held a variety of positions at the FDA, including the Deputy Director of the Division of Anti-Infective Drug Products. Dr. Wikler earned a B.A. in Chemistry from Franklin and Marshall College, an M.D. degree from Temple University School of Medicine, and his M.B.A. from the University of Pennsylvania Wharton School of Business. He completed his Infectious Diseases Fellowship at the Hospital of the University of Pennsylvania and is a Fellow of the Infectious Diseases Society of America. We believe Dr. Wikler is qualified to serve on our board of directors because of his extensive management experience in the pharmaceutical industry and his clinical, drug development and regulatory experience.
There are no family relationships among any of our directors or executive officers.
| 80 |
Scientific Advisory Board
We believe in seeking and attracting scientific and clinical leaders in the field of infectious diseases to provide counsel and support our growth. We have established a Scientific Advisory Board for MAT2203 which consists of individuals who are experts in their chosen fields and recipients of many academic honors and awards.
Board Committees
Our board of directors has three standing committees — an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee.
Audit Committee. The Audit Committee oversees and monitors our financial reporting process and internal control system, reviews and evaluates the audit performed by our registered independent public accountants and reports to the Board any substantive issues found during the audit. The Audit Committee is directly responsible for the appointment, compensation and oversight of the work of our registered independent public accountants. The Audit Committee reviews and approves all transactions with affiliated parties. James Scibetta, Herbert Conrad and Natasha Giordano currently serve as members of the Audit Committee, with Mr. Scibetta serving as its chair. All members of the Audit Committee have been determined to be financially literate and are considered independent directors as defined under the NYSE MKT’s listing standards and applicable SEC rules and regulations. Mr. Scibetta qualifies as an audit committee “financial expert” as that term is defined by SEC regulations. The Audit Committee met four times during 2023. Our Board has adopted an Audit Committee Charter, which is available for viewing at www.matinasbiopharma.com.
Compensation Committee. The Compensation Committee provides advice and makes recommendations to the Board in the areas of employee salaries, benefit programs and director compensation. The Compensation Committee also reviews the compensation of our executive officers, including our chief executive officer, and makes recommendations in that regard to the Board as a whole. Kathryn Corzo, James Scibetta and Matthew Wikler currently serve as members of the Compensation Committee, with Mr. Scibetta serving as its chair. All members of the Compensation Committee are considered independent directors as defined under the NYSE MKT’s listing standards. The Compensation Committee met six times during 2023. Our Board has adopted a Compensation Committee Charter, which is available for viewing at www.matinasbiopharma.com.
Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee nominates individuals to be elected to the full Board by our stockholders. The Nominating and Corporate Governance Committee considers recommendations from stockholders if submitted in a timely manner in accordance with the procedures set forth in our Bylaws and applies the same criteria to all persons being considered. Herbert Conrad, Kathryn Corzo, Eric Ende, and Natasha Giordano currently serve as members of the Nominating and Corporate Governance Committee, with Ms. Giordano serving as its chair. All members of the Nominating and Corporate Governance Committee are considered independent directors as defined under the NYSE MKT’s listing standards. The Nominating and Corporate Governance Committee met four times during 2023. Our Board has adopted a Nominating and Corporate Governance Charter, which is available for viewing at www.matinasbiopharma.com.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers, and employees, including our principal executive officer, principal financial and accounting officer, or persons performing similar functions. A copy of the code is posted on the corporate governance section of our website, which is located at www.matinasbiopharma.com. If we make any substantive amendments to, or grant waivers from, the code of business conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website.
| 81 |
Insider Trading Policy
We have adopted an insider trading policy (the “Trading Policy”) that is designed to promote compliance with federal securities laws, rules and regulations, as well as the rules and regulations of the New York Stock Exchange. The Trading Policy provides Matinas’s standards on trading and causing the trading of our securities or securities of other publicly traded companies while in possession of confidential information. It prohibits trading in certain circumstances and applies to all of our directors, officers and employees as well as independent contractors or consultants who have access to material nonpublic information of Matinas. Additionally, our Trading Policy imposes special additional trading restrictions applicable to all of our directors and executive officers. The Trading Policy is annexed to this Annual Report as an exhibit.
| Item 11. | Executive Compensation |
Summary Compensation Table – 2023
The following table presents information regarding the total compensation awarded to, earned by, or paid to our chief executive officer and the two most highly compensated executive officers who were serving as executive officers as of December 31, 2023 for services rendered in all capacities to us for the years ended December 31, 2023 and 2022. These individuals are our named executive officers for 2023.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) (1) | All Other Compensation ($) | Total ($) | ||||||||||||||||||
| Jerome D. Jabbour | 2023 | 598,000 | 244,375 | 757,176 | - | 1,599,551 | ||||||||||||||||||
| Chief Executive Officer | 2022 | 575,000 | 306,500 | 792,707 | - | 1,674,207 | ||||||||||||||||||
| James J. Ferguson | 2023 | 468,000 | 153,000 | 259,603 | - | 880,603 | ||||||||||||||||||
| Chief Medical Officer | 2022 | 450,000 | 169,920 | 249,179 | - | 869,099 | ||||||||||||||||||
| Theresa Matkovits | 2023 | 430,000 | 140,080 | 281,237 | - | 851,317 | ||||||||||||||||||
| Chief Development Officer | 2022 | 412,000 | 189,000 | 249,179 | - | 850,179 | ||||||||||||||||||
| (1) | Amounts reflect the grant date fair value of option awards granted in 2023 and 2022 in accordance with Accounting Standards Codification Topic 718. These amounts do not correspond to the actual value that will be recognized by the named executive officers. |
Narrative Disclosure to Summary Compensation Table
Employment Agreements with Our Named Executive Officers
Jabbour
On March 22, 2018, we entered into an employment agreement with Mr. Jabbour, as subsequently amended on March 3, 2023. Under the terms of Mr. Jabbour’s employment agreement, Mr. Jabbour received a signing bonus of $84,000 and a base salary of $350,000 per year. Mr. Jabbour’s current salary is $598,000. In addition, Mr. Jabbour is eligible to receive an annual bonus, which is targeted at 50% of his base salary but which may be adjusted by our Compensation Committee based on his individual performance and our performance as a whole. Mr. Jabbour is also eligible to receive option grants at the discretion of our Compensation Committee. Mr. Jabbour is eligible to receive option grants and equity grants at the discretion of our Compensation Committee. If we terminate Mr. Jabbour’s employment without cause or Mr. Jabbour resigns with good reason (absent a change of control), we are required to pay him severance of up to twelve months of his base salary plus COBRA benefits for twelve months, and his target annual bonus for the year pro rated to the date of termination. In addition, the vesting of 50% of his outstanding options issued prior to December 31, 2021 will be accelerated in full upon such termination and Mr. Jabbour will be provided with an extension through two years after the separation date of the exercise period for his vested stock options. If we terminate Mr. Jabbour’s employment without cause during the 24-month period immediately following a change of control or Mr. Jabbour resigns with good reason during the 24-month period immediately following a change of control, we are required to pay him severance of 18 months of his base salary and 1.5 times his target annual bonus plus 18 months of COBRA benefits. In addition, his outstanding options will be vested in full and Mr. Jabbour will be provided with an extension through two years after the separation date of the exercise period for his vested stock options. Mr. Jabbour is also subject to a customary non-disclosure agreement, pursuant to which Mr. Jabbour has agreed to be subject to a non-compete during the term of his employment and for a period of eighteen months following termination of his employment.
| 82 |
Ferguson
On February 22, 2019, we entered into an employment agreement with Mr. Ferguson which was effective as of February 25, 2019, as subsequently amended on March 3, 2023. Under the terms of Mr. Ferguson’s employment agreement, Mr. Ferguson base salary was $375,000 per year, and is currently $468,000. In addition, Mr. Ferguson is eligible to receive an annual bonus, which is targeted at 35% of his base salary but which may be adjusted by our Compensation Committee based on his individual performance and our performance as a whole. Mr. Ferguson is also eligible to receive option grants at the discretion of our Compensation Committee. If we terminate Mr. Ferguson’s employment without cause or Mr. Ferguson resigns with good reason, we are required to pay him severance of up to twelve months of his base salary plus benefits. In addition, the vesting of 50% of his outstanding options issued prior to December 31, 2021 will be accelerated in full upon such termination. If we terminate Mr. Ferguson’s employment without cause during the 12-month period immediately following a change of control or Mr. Jabbour resigns with good reason during the 12-month period immediately following a change of control, we are required to pay him severance of 12 months of his base salary and his target annual bonus plus 12 months of COBRA benefits. In addition, his outstanding options will be vested in full. Mr. Ferguson is also subject to a customary non-disclosure agreement, pursuant to which Mr. Ferguson has agreed to be subject to a non-compete during the term of his employment and for a period of eighteen months following termination of his employment.
Matkovits
On September 25, 2018, we entered into an employment agreement with Ms. Matkovits which was effective as of October 15, 2018, as subsequently amended on March 3, 2023. Under the terms of Ms. Matkovits’ employment agreement, Ms. Matkovits’s base salary was $350,000 per year, and is currently $430,000. In addition, Ms. Matkovits is eligible to receive an annual bonus, which is targeted at 35% of her base salary but which may be adjusted by our Compensation Committee based on her individual performance and our performance as a whole. Ms. Matkovits is also eligible to receive option grants at the discretion of our Compensation Committee. If we terminate Ms. Matkovits’ employment without cause or Ms. Matkovits resigns with good reason, we are required to pay her severance of up to twelve months of his base salary plus benefits. In addition, the vesting of 50% of her outstanding options issued prior to December 31, 2021 will be accelerated in full upon such termination. If we terminate Ms. Matkovits’s employment without cause during the 12-month period immediately following a change of control or Matkovits resigns with good reason during the 12-month period immediately following a change of control, we are required to pay her severance of 12 months of her base salary and her target annual bonus plus 12 months of COBRA benefits. In addition, her outstanding options will be vested in full. Ms. Matkovits is also subject to a customary non-disclosure agreement, pursuant to which Ms. Matkovits has agreed to be subject to a non-compete during the term of her employment and for a period of eighteen months following termination of her employment.
Outstanding Equity Awards at Fiscal Year-End Table – 2023
The following table summarizes, for each of the named executive officers, the number of shares of common stock underlying outstanding stock options held as of December 31, 2023.
| Option Awards | ||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Option exercise price ($) | Option expiration date | ||||||||||||
| Jerome D. Jabbour | - | 3,500,000 | $ | 0.25 | Dec 14, 2033 | |||||||||||
| 538,432 | 1,449,868 | $ | 0.53 | Dec 19, 2032 | ||||||||||||
| 863,558 | 794,542 | $ | 0.92 | Dec 13, 2031 | ||||||||||||
| 1,166,667 | 433,333 | $ | 1.36 | Dec 31, 2030 | ||||||||||||
| 979,167 | 20,833 | $ | 2.27 | Dec 31, 2029 | ||||||||||||
| 750,000 | - | $ | 1.08 | Feb 10, 2029 | ||||||||||||
| 1,000,000 | - | $ | 0.98 | Mar 21, 2028 | ||||||||||||
| 400,000 | - | $ | 3.32 | Feb 20, 2027 | ||||||||||||
| 350,000 | - | $ | 0.43 | Feb 4, 2026 | ||||||||||||
| 175,000 | - | $ | 0.41 | Jan 27, 2025 | ||||||||||||
| 350,000 | - | $ | 1.28 | July 20, 2024 | ||||||||||||
| James J. Ferguson | - | 1,200,000 | $ | 0.25 | Dec 14, 2033 | |||||||||||
| 169,250 | 455,750 | $ | 0.53 | Dec 19, 2032 | ||||||||||||
| 306,029 | 281,571 | $ | 0.92 | Dec 13, 2031 | ||||||||||||
| 419,271 | 155,729 | $ | 1.36 | Dec 31, 2030 | ||||||||||||
| 489,584 | 10,416 | $ | 2.27 | Dec 31, 2029 | ||||||||||||
| 350,000 | - | $ | 1.09 | Feb 24, 2029 | ||||||||||||
| Theresa Matkovits | - | 1,300,000 | $ | 0.25 | Dec 14, 2033 | |||||||||||
| 169,250 | 455,750 | $ | 0.53 | Dec 19, 2032 | ||||||||||||
| 260,406 | 239,594 | $ | 0.92 | Dec 13, 2031 | ||||||||||||
| 309,896 | 115,104 | $ | 1.36 | Dec 31, 2030 | ||||||||||||
| 342,709 | 7,291 | $ | 2.27 | Dec 31, 2029 | ||||||||||||
| 350,000 | - | $ | 1.08 | Feb 10, 2029 | ||||||||||||
| 350,000 | - | $ | 0.79 | Oct 14, 2028 | ||||||||||||
| 83 |
2013 Equity Compensation Plan
General
On August 2, 2013, our Board of Directors adopted the 2013 Equity Compensation Plan pursuant to the terms described herein. The 2013 Equity Compensation Plan was approved by the stockholders on August 7, 2013. Effective May 8, 2014, upon the approval of our Board of Directors and our stockholders, we amended and restated our 2013 Equity Compensation Plan, primarily to include “evergreen” provisions, which provides that the number of shares of common stock available for issuance under the Plan is subject to an automatic annual increase on January 1 of each year beginning in 2015 equal to 4% of the number of shares of common stock outstanding on December 31 of the preceding calendar year or a lesser number of shares of common stock determined by the Board of Directors; to amend the definition of “fair market value”; and to increase the limits on awards under the Plan. The 2013 Equity Compensation Plan, as amended and restated, is referred to herein as the “2013 Plan.”
The general purpose of the 2013 Plan is to provide an incentive to our employees, directors, consultants and advisors by enabling them to share in the future growth of our business. Our Board of Directors believes that the granting of stock options, restricted stock awards, unrestricted stock awards and similar kinds of equity-based compensation promotes continuity of management and increases incentive and personal interest in the welfare of our Company by those who are primarily responsible for shaping and carrying out our long range plans and securing our growth and financial success.
Our Board of Directors believes that the 2013 Plan will advance our interests by enhancing our ability to (a) attract and retain employees, consultants, directors and advisors who are in a position to make significant contributions to our success; (b) reward our employees, consultants, directors and advisors for these contributions; and (c) encourage employees, consultants, directors and advisors to take into account our long-term interests through ownership of our shares.
Description of the 2013 Equity Compensation Plan
The following description of the principal terms of the 2013 Plan is a summary and is qualified in its entirety by the full text of the 2013 Plan, which is attached as Exhibit 10.1 hereto.
| 84 |
Administration. The 2013 Plan will be administered by the Compensation Committee of our Board of Directors, provided that the entire Board of Directors may act in lieu of the Compensation Committee on any matter, subject to certain requirements set forth in the 2013 Plan. The Compensation Committee may grant options to purchase shares of our common stock, stock appreciation rights, stock units, restricted shares of our common stock, performance shares, performance units, incentive bonus awards, other cash-based awards and other stock-based awards. The Compensation Committee also has broad authority to determine the terms and conditions of each option or other kind of award, and adopt, amend and rescind rules and regulations for the administration of the 2013 Plan. Subject to applicable law, the Compensation Committee may authorize one or more reporting persons (as defined in the 2013 Plan) or other officers to make awards (other than awards to reporting persons, or other officers whom the Compensation Committee has specifically authorized to make awards). No awards may be granted under the 2013 Plan on or after the ten year anniversary of the adoption of the 2013 Plan by our Board of Directors, but awards granted prior to such tenth anniversary may extend beyond that date.
Eligibility. Awards may be granted under the 2013 Plan to any person who is an employee, officer, director, consultant, advisor or other individual service provider of the Company or any subsidiary, or any person who is determined by the Compensation Committee to be a prospective employee, officer, director, consultant, advisor or other individual service provider of the Company or any subsidiary.
Shares Subject to the 2013 Plan. As of March 18, 2024, the aggregate number of shares of common stock available for issuance in connection with awards granted under the 2013 Plan is 62,984,400 shares, subject to customary adjustments for stock splits, stock dividends or similar transactions (the “Initial Limit”). Incentive Stock Options may be granted under the 2013 Plan with respect to all of those shares. The number of shares of common stock available for issuance under the 2013 Plan will automatically increase on January 1st of each year for a period of ten years, commencing on January 1, 2015, in an amount equal to four percent (4%) of the total number of shares of common stock outstanding on December 31st of the preceding calendar year (the “Annual Increase”). Notwithstanding the foregoing, the Board of Directors may act prior to the first day of any calendar year, to provide that there shall be no increase in the share reserve for such calendar year or that the Annual Increase in the share reserve for such calendar year shall be a lesser number of shares of common stock than would otherwise occur pursuant to the preceding sentence. The number of shares of common stock which may be issued in respect of Incentive Stock Options is equal to the Current Limit, and will be increased on each January 1, by the Annual Increase for such calendar year.
To the extent that any award under the 2013 Plan payable in shares of common stock is forfeited, cancelled, returned to the Company for failure to satisfy vesting requirements or upon the occurrence of other forfeiture events, or otherwise terminates without payment being made thereunder, the shares of common stock covered thereby will be available for future grants under the 2013 Plan. Shares of common stock that otherwise would have been issued upon the exercise of a stock option or in payment with respect to any other form of award, that are surrendered in payment or partial payment of taxes required to be withheld with respect to the exercise of such stock option or the making of such payment, will also be available for future grants under the 2013 Plan.
Terms and Conditions of Options. Options granted under the 2013 Plan may be either “incentive stock options” that are intended to meet the requirements of Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”) or “nonqualified stock options” that do not meet the requirements of Section 422 of the Code. The Compensation Committee will determine the exercise price of options granted under the 2013 Plan. The exercise price of stock options may not be less than the fair market value, on the date of grant, per share of our common stock issuable upon exercise of the option (or 110% of fair market value in the case of incentive options granted to a ten-percent stockholder).
If on the date of grant the common stock is listed on a stock exchange or national market system, the fair market value shall generally be the closing sale price as of such date, or if there were no trades recorded on such date, then the most recent date preceding such date on which trades were recorded. If on the date of grant the common stock is traded in an over-the-counter market, the fair market will generally be the average of the closing bid and asked prices for the shares of common stock as of such date, or, if there are no closing bid and asked prices for the shares of common stock on such date, then the average of the bid and asked prices for the shares of common stock on the most recent date preceding such date on which such closing bid and asked prices are available. If the common stock is not listed on a national securities exchange or national market system or traded in an over-the-counter market, the fair market value shall be determined by the Compensation Committee in a manner consistent with Section 409A of the Internal Revenue Code of 1986, as amended. Notwithstanding the foregoing, if on the date of grant the common stock is listed on a stock exchange or is quoted on a national market system, or is traded in an over-the-counter market, then solely for purposes of determining the exercise price of any grant of a stock option or the base price of any grant of a stock appreciation right, the Compensation Committee may, in its discretion, base fair market value on the last sale before or the first sale after the grant, the closing price on the trading day before or the trading day of the grant, the arithmetic mean of the high and low prices on the trading day before or the trading day of the grant, or any other reasonable method using actual transactions of the common stock as reported by the exchange or market on which the common stock is traded. In addition, the determination of fair market value also may be made using any other method permitted under Treasury Regulation section 1.409A-1(b)(5)(iv).
| 85 |
No option may be exercisable for more than ten years from the date of grant (five years in the case of an incentive stock option granted to a ten-percent stockholder). Options granted under the 2013 Plan will be exercisable at such time or times as the Compensation Committee prescribes at the time of grant. No employee may receive incentive stock options that first become exercisable in any calendar year in an amount exceeding $100,000. The Compensation Committee may, in its discretion, permit a holder of a nonqualified stock option to exercise the option before it has otherwise become exercisable, in which case the shares of our common stock issued to the recipient will continue to be subject to the vesting requirements that applied to the option before exercise.
Generally, the option price may be paid in cash or by bank check, or such other means as the Compensation Committee may accept. As set forth in an award agreement or otherwise determined by the Compensation Committee, in its sole discretion, at or after grant, payment in full or part of the exercise price of an option may be made (a) in the form of shares of common stock that have been held by the participant for such period as the Compensation Committee may deem appropriate for accounting purposes or otherwise, valued at the fair market value of such shares on the date of exercise; (ii) by surrendering to the Company shares of common stock otherwise receivable on exercise of the option; (iii) by a cashless exercise program implemented by the Compensation Committee in connection with the 2013 Plan; and/or (iv) by such other method as may be approved by the Compensation Committee and set forth in an award agreement.
No option may be transferred other than by will or by the laws of descent and distribution, and during a recipient’s lifetime an option may be exercised only by the recipient or the recipient’s guardian or legal representative. However, the Compensation Committee may permit the transfer of a nonqualified stock option, share-settled stock appreciation right, restricted stock award, performance share or share-settled other stock-based award either (a) by instrument to the participant’s immediate family (as defined in the 2013 Plan), (b) by instrument to an inter vivos or testamentary trust (or other entity) in which the award is to be passed to the participant’s designated beneficiaries, or (c) by gift to charitable institutions. The Compensation Committee will determine the extent to which a holder of a stock option may exercise the option following termination of service.
Stock Appreciation Rights. The Compensation Committee may grant stock appreciation rights independent of or in connection with an option. The Compensation Committee will determine the terms applicable to stock appreciation rights. The base price of a stock appreciation right will be determined by the Compensation Committee, but will not be less than 100% of the fair market value of a share of our common stock with respect to the date of grant of such stock appreciation right. The maximum term of any SAR granted under the 2013 Plan is ten years from the date of grant. Generally, each SAR stock appreciation right will entitle a participant upon exercise to an amount equal to:
| ● | the excess of the fair market value of a share of common stock on the date of exercise of the stock appreciation right over the base price of such stock appreciation right, multiplied by |
| ● | the number of shares as to which such stock appreciation right is exercised. |
Payment may be made in shares of our common stock, in cash, or partly in common stock and partly in cash, all as determined by the Compensation Committee.
Restricted Stock and Stock Units. The Compensation Committee may award restricted common stock and/or stock units under the 2013 Plan. Restricted stock awards consist of shares of stock that are transferred to a participant subject to restrictions that may result in forfeiture if specified conditions are not satisfied. Stock units confer the right to receive shares of our common stock, cash, or a combination of shares and cash, at a future date upon or following the attainment of certain conditions specified by the Compensation Committee. The Compensation Committee will determine the restrictions and conditions applicable to each award of restricted stock or stock units, which may include performance-based conditions. Dividends with respect to restricted stock may be paid to the holder of the shares as and when dividends are paid to stockholders or at the times of vesting or other payment of the restricted stock award. Stock unit awards may be granted with dividend equivalent rights, which may be accumulated and may be deemed reinvested in additional stock units, as determined by the Compensation Committee in its discretion. If any dividend equivalents are paid while a stock unit award is subject to restrictions, the dividend equivalents shall be subject to the same restrictions on transferability as the underlying stock units, unless otherwise set forth in an award agreement. Unless the Compensation Committee determines otherwise, holders of restricted stock will have the right to vote the shares.
| 86 |
Performance Shares and Performance Units. The Compensation Committee may award performance shares and/or performance units under the 2013 Plan. Performance shares and performance units are awards which are earned during a specified performance period subject to the attainment of performance criteria, as established by the Compensation Committee. The Compensation Committee will determine the restrictions and conditions applicable to each award of performance shares and performance units.
Incentive Bonus Awards. The Compensation Committee may award Incentive Bonus Awards under the 2013 Plan. Incentive Bonus Awards may be based upon the attainment of specified levels of Company or subsidiary performance as measured by pre-established, objective performance criteria determined at the discretion of the Compensation Committee. Incentive Bonus Awards will be paid in cash or common stock, as set forth in an award agreement.
Other Stock-Based and Cash-Based Awards. The Compensation Committee may award other types of equity-based or cash-based awards under the 2013 Plan, including the grant or offer for sale of unrestricted shares of our common stock and payment in cash or otherwise of amounts based on the value of shares of common stock.
Effect of Certain Corporate Transactions. The Compensation Committee may, at the time of the grant of an award, provide for the effect of a change in control (as defined in the 2013 Plan) on any award, including (i) accelerating or extending the time periods for exercising, vesting in, or realizing gain from any award, (ii) eliminating or modifying the performance or other conditions of an award, (iii) providing for the cash settlement of an award for an equivalent cash value, as determined by the Compensation Committee, or (iv) such other modification or adjustment to an award as the Compensation Committee deems appropriate to maintain and protect the rights and interests of participants upon or following a change in control. The Compensation Committee may, in its discretion and without the need for the consent of any recipient of an award, also take one or more of the following actions contingent upon the occurrence of a change in control: (a) cause any or all outstanding options and stock appreciation rights to become immediately exercisable, in whole or in part; (b) cause any other awards to become non-forfeitable, in whole or in part; (c) cancel any option or stock appreciation right in exchange for a substitute option; (d) cancel any award of restricted stock, stock units, performance shares or performance units in exchange for a similar award of the capital stock of any successor corporation; (e) redeem any restricted stock, stock unit, performance share or performance unit for cash and/or other substitute consideration with a value equal to the fair market value of an unrestricted share of our common stock on the date of the change in control; (f) cancel any option or stock appreciation right in exchange for cash and/or other substitute consideration based on the value of our common stock on the date of the change in control , and cancel any option or stock appreciation right without any payment if its exercise price exceeds the value of our common stock on the date of the change in control; (g) cancel any stock unit or performance unit held by a participant affected by the change in control in exchange for cash and/or other substitute consideration with a value equal to the fair market value per share of common stock on the date of the change in control, or (h) make such other modifications, adjustments or amendments to outstanding awards as the Compensation Committee deems necessary or appropriate.
Amendment, Termination. The Compensation Committee may amend the terms of awards in any manner not inconsistent with the 2013 Plan, provided that no amendment shall adversely affect the rights of a participant with respect to an outstanding award without the participant’s consent. In addition, our board of directors may at any time amend, suspend, or terminate the 2013 Plan, provided that (i) no such amendment, suspension or termination shall materially and adversely affect the rights of any participant under any outstanding award without the consent of such participant and (ii) to the extent necessary and desirable to comply with any applicable law, regulation, or stock exchange rule, the 2013 Plan requires us to obtain stockholder consent. Stockholder approval is required for any plan amendment that increases the number of shares of common stock available for issuance under the 2013 Plan or changes the persons or classes of persons eligible to receive awards.
| 87 |
Tax Withholding
The Company has the power and right to deduct or withhold, or require a participant to remit to the Company, the minimum statutory amount to satisfy federal, state, and local taxes, domestic or foreign, required by law or regulations to be withheld.
Director Compensation
We maintain a policy pursuant to which our non-employee directors receive annualized compensation. The policy provides for the following compensation amounts payable in cash, or upon election by such non-employee director, in shares of unrestricted common stock: (i) each non-employee director is entitled to receive an annual fee of $50,000; (ii) the chairman of the board is entitled to receive an additional annual fee of $25,000; (iii) the vice chair, if one is appointed, is entitled to receive an additional annual fee of $20,000; (iv) the chair of our audit committee is entitled to receive an annual fee of $15,000 and other members of our audit committee are entitled to receive $7,500; (v) the chair of our compensation committee is entitled to receive an annual fee of $10,000 and other members of our compensation committee are entitled to receive $6,000; and (vi) the chair of our nominating and corporate governance committee is entitled to receive an annual fee of $8,000 and other members are entitled to receive $4,000.
As of the date of each annual meeting of the shareholders, each non-employee director will receive an option grant to purchase shares of our common stock valued at $80,000 as determined by the Black Scholes method on the date of grant under our existing equity incentive plan, or any other equity incentive plan we may adopt in the future, which shall vest in twelve equal monthly installments.
All fees under the director compensation policy are paid on a quarterly basis in arrears and no per meeting fees are paid. All fees may be paid in unrestricted shares of common stock at the election of the director. We also reimburse non-employee directors for reasonable expenses incurred in connection with attending board of director and committee meetings.
Director Compensation Table – 2023
The following table summarizes the annual compensation for our non-employee directors during 2023.
| Name | Cash Compensation ($) | Option Awards ($) (1) | Total ($) | |||||||||
| Herbert Conrad | 61,500 | 80,000 | 141,500 | |||||||||
| Kathryn Corzo | 60,000 | 80,000 | 140,000 | |||||||||
| Eric Ende | 79,000 | 80,000 | 159,000 | |||||||||
| Natasha Giordano | 65,500 | 80,000 | 145,500 | |||||||||
| James S. Scibetta | 75,000 | 80,000 | 155,000 | |||||||||
| Matthew Wikler | 56,000 | 80,000 | 136,000 | |||||||||
| (1) | Amounts reflect the grant date fair value of stock awards and option awards granted in 2023 in accordance with Accounting Standards Codification Topic 718. These amounts do not correspond to the actual value that will be recognized by the directors. |
Compensation Committee Interlocks and Insider Participation
The Compensation Committee of the Board of Directors is currently composed of the following three non-employee directors: Kathryn Corzo, James Scibetta, Chair, and Matthew Wikler. No member of the Compensation Committee is or was formerly an officer or an employee of the Company during the last fiscal year. In addition, no executive officer of the Company serves on the compensation committee or board of directors of a company for which any of the Company’s directors serve as an executive officer. See Item 13.
| 88 |
| Item 12. | Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters. |
The following table sets forth the number of shares of common stock beneficially owned as of March 18, 2024 by:
| ● | each of our stockholders who is known by us to beneficially own 5% or more of our common stock; |
| ● | each of our executive officers; |
| ● | each of our directors; and |
| ● | all of our directors and current executive officers as a group. |
Beneficial ownership is determined based on the rules and regulations of the SEC. A person has beneficial ownership of shares if such individual has the power to vote and/or dispose of shares. This power may be sole or shared and direct or indirect. Applicable percentage ownership in the following table is based on 217,482,830 shares outstanding as of March 18, 2024. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock that are subject to options or warrants held by that person and exercisable as of, or within 60 days of, March 18, 2024 are counted as outstanding. These shares, however, are not counted as outstanding for the purposes of computing the percentage ownership of any other person(s). Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of common stock set forth opposite that person’s name. Unless indicated below, the address of each individual listed below is c/o Matinas BioPharma Holdings, Inc., 1545 Route 206 South, Suite 302, Bedminster, NJ 07921.
| Name of Beneficial Owner | Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | |||||||
| Directors and Executive Officers | |||||||||
| Jerome D. Jabbour (1) | 6,912,028 | 3.1 | % | ||||||
| Herbert Conrad (2) | 6,057,976 | 2.8 | % | ||||||
| Kathryn Corzo (3) | 549,186 | * | % | ||||||
| Eric Ende (4) | 1,519,169 | * | % | ||||||
| Natasha Giordano (5) | 776,515 | * | % | ||||||
| James Scibetta (6) | 1,937,535 | * | % | ||||||
| Matthew Wikler (7) | 1,515,549 | * | % | ||||||
| James J. Ferguson (8) | 1,905,501 | * | % | ||||||
| Thomas J. Hoover (9) | 681,758 | * | % | ||||||
| Keith A. Kucinski (10) | 1,909,578 | * | % | ||||||
| Hui Liu (11) | 894,770 | * | % | ||||||
| Theresa Matkovits (12) | 1,927,578 | * | % | ||||||
| Directors and Executive Officers as a group (12 persons) (13) | 26,587,143 | 11.2 | % | ||||||
* Less than 1%
(1) Includes 6,451,704 shares of common stock issuable upon exercise of options. Does not include 5,707,196 shares of common stock underlying options.
(2) Includes 1,363,410 shares of common stock issuable upon exercise of options. Does not include 182,185 shares of common stock underlying options.
(3) Includes 549,186 shares of common stock issuable upon exercise of options. Does not include 212,478 shares of common stock underlying options.
(4) Includes 1,375,077 shares of common stock issuable upon exercise of options. Does not include 182,185 shares of common stock underlying options.
(5) Includes 776,515 shares of common stock issuable upon exercise of. Does not include 182,185 shares of common stock underlying option.
(6) Includes 1,287,577 shares of common stock issuable upon exercise of options. Does not include 182,185 shares of common stock underlying options.
(7) Includes 1,225,077 shares of common stock issuable upon exercise of options. Does not include 182,185 shares of common stock underlying options.
(8) Includes 1,905,501 shares of common stock issuable upon exercise of options. Does not include 1,932,099 shares of common stock underlying options.
(9) Includes 691,758 shares of common stock issuable upon exercise of options. Does not include 1,643,242 shares of common stock underlying options.
(10) Includes 1,815,078 shares of common stock issuable upon exercise of options. Does not include 1,684,922 shares of common stock underlying options.
(11) Includes 894,770 shares of common stock issuable upon exercise of options. Does not include 1,605,230 shares of common stock underlying options.
(12) Includes 1,927,578 shares of common stock issuable upon exercise of options. Does not include 1,972,422 shares of common stock underlying options.
(13) See notes (1) through (12).
| 89 |
Securities Authorized for Issuance under Equity Compensation Plans
The following table summarizes information about our equity compensation plans as of December 31, 2023.
| Plan Category | Number of Shares of Common Stock to be Issued upon Exercise of Outstanding Options (a) | Weighted-Average Exercise Price of Outstanding Options (b) | Number of Options Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (c)(2) | |||||||||
| Equity compensation plans approved by stockholders(1) | 46,707,934 | $ | 0.83 | 2,905,170 | ||||||||
| Equity compensation plans not approved by stockholders | — | — | — | |||||||||
| Total | 46,707,934 | $ | 0.83 | 2,905,170 | ||||||||
| (1) | The amounts shown in this row include securities under the Matinas BioPharma Holdings, Inc. Amended and Restated 2013 Equity Incentive Plan (the “2013 Plan”). |
| (2) | In accordance with the “evergreen” provision in our 2013 Plan, an additional 8,690,581 shares were automatically made available for issuance on the first trading day of 2024, which represents 4% of the number of shares outstanding on December 31, 2023; these shares are excluded from this calculation. |
| Item 13. | Certain Relationships, Related Transactions, And Director Independence |
Certain Relationships and Related Party Transactions
Other than compensation arrangements for our named executive officers and directors, there has been no transaction or series of similar transactions, since January 1, 2023, to which we were a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed the lesser of (i) $120,000 and (ii) one percent of the average of our total assets at year-end for the last two completed fiscal years; and |
| ● | any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Indemnification Agreements
We entered into indemnification agreements with our directors and executive officers. The indemnification agreements provide for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The indemnification agreements also provide for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The indemnification agreement set forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that apply to any dispute between us and an indemnitee arising under the Indemnification Agreements.
Policies and Procedures for Related Party Transactions
We have adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest, which we refer to collectively as related parties, are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the audit committee or, in certain circumstances, the chairman of the audit committee. Any request for us to enter into a transaction with a related party, in which the amount involved exceeds $100,000 and such related party would have a direct or indirect interest must first be presented to our audit committee, or in certain circumstances the chairman of our audit committee, for review, consideration and approval. In approving or rejecting any such proposal, our audit committee, or the chairman of our audit committee, is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related party’s interest in the transaction.
Director Independence
Based on information requested from and provided by each of our directors, our board of directors has determined that Messrs. Herbert Conrad, Eric Ende, James Scibetta, Matthew Wikler and Mses. Kathryn Corzo and Natasha Giordano are “independent directors” as such term is defined in the rules of the NYSE MKT’s corporate governance requirements and Rule 10A-3 promulgated under the Securities Exchange Act of 1934, as amended.
| 90 |
| Item 14. | Principal Accounting Fees And Services |
The following table represents aggregate fees billed to the Company for the fiscal years ended December 31, 2023 and 2022, by EisnerAmper LLP, the Company’s independent registered public accounting firm.
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Audit Fees | $ | 270 | $ | 265 | ||||
| Audit-Related Fees | - | - | ||||||
| Tax Fees | - | - | ||||||
| Total Fees | $ | 270 | $ | 265 | ||||
Audit Fees consist of fees billed for professional services rendered for the audit of our annual financial statements, audit of internal controls over financial reporting, review of our interim consolidated financial statements, comfort and consent letters.
Audit-Related Fees consist of fees billed for professional services rendered for assurance related services that are reasonably related to the performance of the audit or review of our financial services.
Tax Fees are for tax-related services related primarily to tax consulting and tax planning.
The Audit Committee pre-approves all auditing services and any non-audit services that the independent registered public accounting firm is permitted to render under Section 10A (h) of the Exchange Act. The Audit Committee may delegate the pre-approval to one of its members, provided that if such delegation is made, the full Audit Committee must be presented at its next regularly scheduled meeting with any pre-approval decision made by that member.
| 91 |
Part IV
| Item 15. | Exhibits And Financial Statement Schedules |
| 92 |
| + | Confidential treatment has been requested for certain provisions of this Exhibit pursuant to Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended. |
| † | Indicates a management contract or compensation plan, contract or arrangement. |
| * | Filed herewith. |
| ** | Furnished herewith. |
| Item 16. | Form 10-K Summary. |
None.
| 93 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Bedminster, State of New Jersey on March 27, 2024.
| MATINAS BIOPHARMA HOLDINGS, INC. | ||
| By: | /s/ Jerome D. Jabbour | |
| Name: | Jerome D. Jabbour | |
| Title: | Chief Executive Officer | |
| By: | /s/ Keith A. Kucinski | |
| Name: | Keith A. Kucinski | |
| Title: | Chief Financial Officer | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Person | Capacity | Date | ||
| /s/ Jerome D. Jabbour | Chief Executive Officer and Director | March 27, 2024 | ||
| Jerome D. Jabbour | (Principal Executive Officer) | |||
| /s/ Keith A. Kucinski | Chief Financial Officer | March 27, 2024 | ||
| Keith A. Kucinski | (Principal Financial and Accounting Officer) | |||
| /s/ Eric Ende | Chairman of the Board | March 27, 2024 | ||
| Eric Ende | ||||
| /s/ Herbert Conrad | Director | March 27, 2024 | ||
| Herbert Conrad | ||||
| /s/ Kathryn Corzo | Director | March 27, 2024 | ||
| Kathryn Corzo | ||||
| /s/ Natasha Giordano | Director | March 27, 2024 | ||
| Natasha Giordano | ||||
| /s/ James S. Scibetta | Director | March 27, 2024 | ||
| James S. Scibetta | ||||
| /s/ Matthew A. Wikler | Director | March 27, 2024 | ||
| Matthew A. Wikler |
| 94 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
Matinas BioPharma Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Matinas BioPharma Holdings, Inc. and Subsidiaries as of December 31, 2023 and 2022, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of their operations and their cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has recurring net losses and net cash flow used in operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| F-1 |
Accruals and prepaid balance for research and development expenses
As disclosed in the consolidated statements of operations, for the year ended December 31, 2023, the Company incurred significant research and development (“R&D”) expenses, which amounted to approximately $14.5 million. At December 31, 2023, the Company had accrued approximately $0.9 million for R&D expenses on the consolidated balance sheet. The Company also recorded prepaid R&D expense of approximately $0.7 million on the consolidated balance sheet. A significant amount of the Company’s R&D expenses are service fees paid to contract research organizations (“CROs”) and contract manufacturing organizations (“CMOs”). The R&D activities with these CROs and CMOs are documented in contractual agreements and are typically performed over an extended period. The amounts recorded on the consolidated balance sheet for such R&D contracts represent the Company’s estimates of the unpaid and prepaid R&D expenses based on facts and circumstances known to the Company at that time, and are dependent upon the timely and accurate reporting of CROs and CMOs. The amount of R&D expense to be recognized under these agreements based on the Company’s estimate of the progress of work completed during the financial reporting period and involves judgement and estimation.
We identified management’s estimate of accruals and prepaid balances for R&D expenses as a critical audit matter due to the significance of these expenses to the financial statements and the significant judgement and estimation required by management in determining the progress or state of completion of clinical studies or services rendered. As a result, a high degree of auditor judgement and additional testing were required to evaluate audit evidence relating to estimates made by management.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. Our audit procedures related to accruals and prepaid balances for R&D expenses included the following, among others, (i) we obtained an understanding of management’s process and evaluated the design and implementation of controls over developing its estimate of accrued and prepaid R&D expenses, including the process of estimating the expenses incurred to date based on the status of the clinical studies; (ii) we read selected agreements and contract amendments with CROs and CMOs and evaluating the significant assumptions described above and the methods used in developing the R&D estimates and recalculating the amounts that were unpaid and prepaid at the balance sheet date, (iii) we confirmed with certain CROs and CMOs contractual commitments and amounts completed, paid and unpaid, and (iv) made direct inquiries of the Company research personnel regarding project status, and inspected invoices received subsequent to year-end and additional documents and correspondence with the CROs and CMOs when used by management to develop its estimate of R&D expenditures.
/s/ EisnerAmper LLP
We have served as the Company’s auditor since 2011
March 27, 2024
| F-2 |
Matinas BioPharma Holdings, Inc.
Consolidated Balance Sheets
(in thousands, except for share data)
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| ASSETS: | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Marketable debt securities | ||||||||
| Restricted cash – security deposit | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Non-current assets: | ||||||||
| Leasehold improvements and equipment - net | ||||||||
| Operating lease right-of-use assets - net | ||||||||
| Finance lease right-of-use assets - net | ||||||||
| In-process research and development | ||||||||
| Goodwill | ||||||||
| Restricted cash - security deposit | ||||||||
| Total non-current assets | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY: | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses and other liabilities | ||||||||
| Operating lease liabilities - current | ||||||||
| Financing lease liabilities - current | ||||||||
| Total current liabilities | ||||||||
| Non-current liabilities: | ||||||||
| Deferred tax liability | ||||||||
| Operating lease liabilities - net of current portion | ||||||||
| Financing lease liabilities - net of current portion | ||||||||
| Total non-current liabilities | ||||||||
| Total liabilities | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock par value $ per share, shares authorized at December 31, 2023 and 2022, respectively; issued and outstanding as of December 31, 2023 and 2022, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
Matinas BioPharma Holdings, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
| For the Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenue: | ||||||||
| Contract Revenue | $ | $ | ||||||
| Costs and Expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total costs and expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Sale of New Jersey net operating loss & tax credits | ||||||||
| Other income, net | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Net loss per share – basic and diluted | $ | ) | $ | ) | ||||
| Weighted average common shares outstanding: | ||||||||
| Basic and diluted | ||||||||
| Other comprehensive gain/(loss), net of tax | ||||||||
| Unrealized gain/(loss) on securities available-for-sale | ( | ) | ||||||
| Other comprehensive gain/(loss), net of tax | ( | ) | ||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
Matinas BioPharma Holdings, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
(in thousands, except for share data)
| Common Stock | Additional Paid-in | Accumulated | Accumulated Other Comprehensive | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | (Loss)/Income | Equity | |||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||
| Issuance of common stock upon exercise of options | ||||||||||||||||||||||||
| Issuance of common stock in exchange for warrants | ||||||||||||||||||||||||
| Issuance of common stock pursuant to license agreement amendment | ||||||||||||||||||||||||
| Other comprehensive loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||
| Other comprehensive income | — | |||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
Matinas BioPharma Holdings, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| For the Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Loss on disposal of equipment | ||||||||
| Stock-based compensation expense | ||||||||
| Amortization of operating lease right-of-use assets | ||||||||
| Amortization of finance lease right-of-use assets | ||||||||
| Amortization of bond discount | ||||||||
| Stock issued pursuant to license agreement amendment | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | ( | ) | ||||||
| Accounts payable | ( | ) | ( | ) | ||||
| Accrued expenses and other liabilities | ( | ) | ||||||
| Operating lease liabilities | ( | ) | ( | ) | ||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of marketable debt securities | ( | ) | ||||||
| Proceeds from sales of marketable debt securities | ||||||||
| Purchases of leasehold improvements and equipment | ( | ) | ( | ) | ||||
| Net cash provided by investing activities | ||||||||
| Cash flows from financing activities: | ||||||||
| Proceeds from exercise of options | ||||||||
| Payments of capital lease liability – principal | ( | ) | ( | ) | ||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||
| Net decrease in cash, cash equivalents and restricted cash | ( | ) | ( | ) | ||||
| Cash, cash equivalents and restricted cash at beginning of period | ||||||||
| Cash, cash equivalents and restricted cash at end of period | $ | $ | ||||||
| Supplemental non-cash financing and investing activities: | ||||||||
| Unrealized gain/(loss) on marketable debt securities | $ | $ | ( | ) | ||||
| Cash exercise of warrants in prepaid expenses and other current assets | $ | $ | ( | ) | ||||
| Right-of-use asset in exchange from liabilities from an operating lease modification | $ | $ | ( | ) | ||||
| Right-of-use asset in exchange from liabilities from finance leases | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
Note 1 – Description of Business
Matinas BioPharma Holdings Inc. (“Holdings”) is a Delaware corporation formed in 2013. Holdings is the parent company of Matinas BioPharma, Inc. (“BioPharma”), and Matinas BioPharma Nanotechnologies, Inc. (“Nanotechnologies,” formerly known as Aquarius Biotechnologies, Inc.), its operating subsidiaries (“Nanotechnologies”, and together with “Holdings” and “BioPharma”, “the Company”). The Company is a clinical-stage biopharmaceutical company with a focus on identifying and developing novel pharmaceutical products.
Note 2 – Liquidity, Plan of Operations and Going Concern
The
Company has experienced net losses and negative cash flows from operations each period since its inception. Through December 31, 2023,
the Company had an accumulated deficit of $
The Company has been engaged in developing its lipid nanocrystal (“LNC”) platform delivery technology and a pipeline of associated product candidates, including MAT2203 and MAT2501, since 2011. To date, the Company has not obtained regulatory approval for any of its product candidates nor generated any revenue from product sales, and the Company expects to incur significant expenses to complete development of its product candidates. The Company may never be able to obtain regulatory approval for the marketing of any of its product candidates in any indication in the United States or internationally and there can be no assurance that the Company will generate revenues or ever achieve profitability.
If the Company obtains U.S. Food and Drug Administration (“FDA”) approval for one or more of its product candidates, the Company expects that its expenses will continue to increase once the Company reaches commercial launch. The Company also expects that its research and development expenses will continue to increase as it moves forward with additional clinical studies for its current product candidates and development of additional product candidates. As a result, the Company expects to continue to incur substantial losses for the foreseeable future, and that these losses will be increasing.
As
of December 31, 2023, the Company had cash and cash equivalents of $
The ability of the Company to continue as a going concern is dependent upon control over its operating expenses, anticipated proceeds from future sales of common stock through the At-The-Market Sales Agreement (“Sales Agreement”) with BTIG, LLC. and securing additional financing. While the Company believes in the viability of this strategy and believes the actions presently being taken by the Company provide the opportunity for it to continue as a going concern, there can be no assurance the Company will be successful in its implementation. In particular, utilization of the Sales Agreement may not be viable due to market conditions and new financing may not be available on acceptable terms, or at all. These consolidated financial statements do not include any adjustments related to the recoverability and classification of asset amounts or the amounts and classification of liabilities that might be necessary if the Company is unable to continue as a going concern.
Note 3 – Summary of Significant Accounting Policies
Basis of presentation and principles of consolidation
The accompanying consolidated financial statements include the consolidated accounts of Holdings and its wholly owned subsidiaries, BioPharma, and Nanotechnologies. The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) and reflect the operations of the Company and its wholly owned subsidiaries. All intercompany transactions have been eliminated in consolidation.
| F-7 |
Financial impact of events beyond our control
Our financial condition and results of operations may be impacted by factors we may not be able to control, such as pandemics, global supply chain disruptions, global trade disputes and/or political instability. Increases in interest rates, especially if coupled with reduced government spending and volatility in financial markets, may have the effect of further increasing economic uncertainty and heightening these risks. Additionally, rising inflation rates may affect us by increasing operating expenses, such as employee-related costs and clinical trial expenses, negatively impacting our results of operations.
The Company’s financial results for the year ended December 31, 2023 were not significantly impacted by factors beyond our control, such as those described above. However, the Company cannot predict the impact of any of these factors on future results or the Company’s ability to raise capital due to a variety of factors, including but not limited to the continued good health of Company employees, the ability of service providers and suppliers to continue to operate and deliver, the ability of the Company to maintain operations, and any government and/or public actions taken in response to these factors.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Significant items subject to such estimates and assumptions include, but are not limited to, the Company’s research and development expenses and the assessment of the impairment of goodwill and intangible assets.
Segment and geographic information
Operating
segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief
operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views
its operations and manages its business in
Cash, cash equivalents and restricted cash
The Company considers all highly liquid financial instruments with original maturities of three months or less when purchased to be cash and cash equivalents and all investments with maturities of greater than three months from date of purchase are classified as marketable debt securities. Cash and cash equivalents consisted of cash in bank checking and savings accounts, money market funds and short-term U.S. treasury bonds that mature within three months of settlement date. The Company presents restricted cash with cash and cash equivalents in the Consolidated Statements of Cash Flows. Restricted cash represents funds the Company is required to set aside to cover building operating leases and other purposes. For a complete disclosure of the Company’s cash, cash equivalents and restricted cash, see Note 4 – Cash, Cash Equivalents, Restricted Cash and Marketable Debt Securities.
Marketable Debt Securities
Marketable debt securities, all of which are available-for-sale, consist of U.S. treasury bonds, U.S. government notes and corporate debt securities are carried at fair value, with unrealized gains and losses reported as accumulated other comprehensive loss, except for losses from impairments which are determined to be other-than-temporary. Any premium or discount arising at purchase is amortized and/or accreted to interest income and/or expense over the life of the instrument. The Company reviews its portfolio of available-for-sale debt securities, using both qualitative and quantitative factors, to determine if declines in fair value below cost have resulted from a credit-related loss or other factors. Realized gains and losses and declines in value judged to be other-than-temporary are included in the determination of net loss and are included in other income, net. Fair values are based on quoted market prices at the reporting date. Interest and dividends on available-for-sale securities are included in other income, net. For a complete disclosure of the Company’s marketable debt securities, see Note 4 – Cash, Cash Equivalents, Restricted Cash and Marketable Debt Securities.
| F-8 |
Concentration of credit risk
The
Company’s financial instruments that are exposed to concentrations of credit risk consist primarily of cash, cash equivalents,
restricted cash and marketable debt securities. The Company’s investment policy is to invest only in institutions that meet high
credit quality standards and establishes limits on the amount and time to maturity of investments with any individual counterparty. Balances
are maintained at U.S. financial institutions and may from time to time exceed the Federal Deposit Insurance Corporation (“FDIC”)
insurance limit of $
Leasehold improvements and equipment
Leasehold
improvements and equipment are stated at cost less accumulated depreciation and amortization. Depreciation on equipment is computed using
the straight-line method over the estimated useful lives of the assets, which range from to
Upon retirement or sale, the cost of assets disposed of and related accumulated depreciation and amortization is removed from the accounts and any resulting gain or loss is included in loss from operations. Expenditures for repairs and maintenance are charged to expense as incurred. For a complete disclosure of the Company’s leasehold improvements and equipment, see Note 6 – Leasehold Improvements and Equipment.
Goodwill and other intangible assets
Goodwill is recorded when consideration paid for an acquired entity exceeds the fair value of the net assets acquired. Goodwill is not amortized but rather is assessed for impairment at least annually on a reporting unit basis, or more frequently when events and circumstances indicate the goodwill may be impaired. U.S. GAAP provides that the Company has the option to perform a qualitative assessment to determine whether it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount. If the Company determines this is the case, the Company can perform further quantitative analysis to identify and measure the amount of goodwill impairment loss to be recognized, if any.
A reporting unit is an operating segment, or one level below an operating segment. Historically, the Company has conducted its business in a single operating segment and reporting unit.
For the year ended December 31, 2023, due primarily to there being substantial doubt about the ability of the Company to continue as a going concern, the Company assessed goodwill impairment by performing a quantitative analysis for its reporting unit. As part of the quantitative review, the Company considered whether its fair value, determined as the price a market participant would be willing to pay in a potential acquisition of the Company, exceeds its carrying value, including goodwill. Based on the results of the Company’s assessment, it was determined that its goodwill was not impaired.
For the year ended December 31, 2022, the Company assessed goodwill impairment by performing a qualitative analysis for its reporting unit. As part of the qualitative review, the Company considered relevant events and circumstances, with an emphasis on the fact that the Company’s fair value, as determined by its market capitalization, was well in excess of its book value. Based on the results of the Company’s assessment, it was determined that it is more-likely-than-not that its goodwill was not impaired.
Indefinite lived intangible assets are composed of in-process research and development (“IPR&D”) and represent projects acquired in a business combination that have not reached technological feasibility or that lack regulatory approval at the time of acquisition. These IPR&D assets are reviewed for impairment annually, or sooner if events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable, and upon establishment of technological feasibility or regulatory approval. An impairment loss, if any, is calculated by comparing the fair value of the asset to its carrying value. If the asset’s carrying value exceeds its fair value, an impairment loss is recorded for the difference and its carrying value is reduced accordingly. Similar to the impairment test for goodwill, the Company may perform a qualitative review of its indefinite-lived intangible assets for impairment in order to determine the necessity of performing a quantitative analysis to identify and measure the amount of impairment loss to be recognized, if any.
| F-9 |
For the year ended December 31, 2023, the Company performed a quantitative analysis, using a discounted cash flow approach to determine fair value, and concluded that its indefinite-lived assets were not impaired. For the year ended December 31, 2022, the Company used the qualitative approach and concluded that it was more-likely-than-not that its indefinite-lived assets were not impaired.
Leases
The Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 842, “Leases”, establishes a right-of-use (“ROU”) model that requires a lessee to recognize a ROU asset and lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement. Lessor accounting under the new standard is substantially unchanged. Additional qualitative and quantitative disclosures are also required. For a complete disclosure of the Company’s leases, see Note 8 – Leases.
Income taxes
Deferred taxes are provided on a liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carry forwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates in the period that includes the enactment date.
The
Company adopted the provisions of ASC 740-10 and has analyzed its filing positions in 2023 and 2022 in jurisdictions where it may be
obligated to file returns. The Company believes that its income tax filing position and deductions will be sustained on audit and does
not anticipate any adjustments that will result in a material change to its financial position. Therefore, no reserves for uncertain
income tax positions have been recorded. The Company’s policy is to recognize interest and/or penalties related to income tax matters
in income tax expense. The Company had
Since the Company incurred net operating losses in every tax year since inception, the 2014 through 2022 income tax returns are subject to examination and adjustments by the Internal Revenue Service for at least three years following the year in which the tax attributes are utilized.
Fair Value Measurements
As defined in ASC 820 “Fair Value Measurement”, fair value measurements should be disclosed separately by three levels of the fair value hierarchy. For assets and liabilities recorded at fair value, it is the Company’s policy to maximize the use of observable inputs (quoted prices in active markets) and minimized the use of unobservable inputs (the Company’s assumptions) when developing fair value measurements, in accordance with the established fair value hierarchy. For a complete disclosure of the Company’s fair value measurements, see Note 5 – Fair Value Measurements.
Stock-based compensation to employees consist of stock option grants and restricted shares. The Company accounts for stock-based compensation under the provisions of ASC 718-10, Compensation – Stock Compensation, which requires all share-based payments to employees, non-employees and directors, including grants of stock options and restricted shares, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values on the date of grant over the requisite service period, which is generally the vesting period of the respective award. Forfeitures are accounted for as they occur. Generally, the Company issues stock option awards with only service-based vesting conditions and records the expense for these awards using the straight-line method. The Company classifies stock-based compensation expense in the same manner in which the awards recipient’s payroll or service provider’s costs are classified.
| F-10 |
The Company accounts for equity instruments issued to non-employees in accordance with the provisions of ASC Topic 505, subtopic 50, Equity-Based Payments to Non-Employees based upon the fair-value of the underlying instrument. The equity instruments, consisting of stock options granted to consultants, are valued using the Black-Scholes valuation model. The Company calculates the fair value of option grants utilizing the Black-Scholes pricing model and estimates the fair value of restricted stock based upon the estimated fair value or the common stock.
The resulting compensation expense for both employee and non-employee awards is generally recognized on a straight-line basis over the requisite service period of the award.
Net loss per share information is determined using the two-class method, which includes the weighted-average number of shares of common stock outstanding during the period and other securities.
Under the two-class method, basic net loss per share attributable to common stockholders is computed by dividing the net income attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share attributable to common stockholders is computed using the more dilutive of (1) the two-class method or (2) the if-converted method.
During the years ended December 31, 2023 and 2022, diluted earnings per common share is the same as basic earnings per common share because, as the Company incurred a net loss during each period presented, the potentially dilutive securities from the assumed exercise of all outstanding stock options and warrants would have an anti-dilutive effect. The reconciliation of the diluted shares as of December 31, 2023 and 2022 are as follows:
| As of December 31, | ||||||||
| 2023 | 2022 | |||||||
| Stock options | ||||||||
| Warrants | ||||||||
| Total | ||||||||
Revenue recognition
Pursuant to Topic 606, the Company recognizes revenue to depict the transfer of promised goods or services to a customer in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this core principle, Topic 606 outlines a five-step process for recognizing revenue from customer contracts that includes i) identification of the contract with a customer, ii) identification of the performance obligations in the contract, iii) determining the transaction price, iv) allocating the transaction price to the separate performance obligations in the contract, and v) recognizing revenue associated with performance obligations as they are satisfied.
At contract inception, the Company assesses the goods or services promised within each contract and assess whether each promised good or service is distinct and determine those that are performance obligations. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when the performance obligation is satisfied.
For the years ended December 31, 2023 and 2022, the Company’s revenues primarily consist of contract research revenue from the BioNTech Agreement to evaluate the combination of mRNA formats utilizing the Company’s proprietary LNC Platform. For a complete disclosure of the Company’s Revenue Recognition, see Note 9 – Revenue Recognition, Collaboration Agreements, and Other Research and Development Agreements.
On
December 12, 2019, the Company entered into a feasibility study agreement (the “Agreement”) with Genentech, Inc. (“Genentech”).
This feasibility study involves the development of oral formulations using the Company’s LNC Platform, which enables the development
of a wide range of difficult-to-deliver molecules. Under the terms of the Agreement, Genentech paid the Company a total of $
| F-11 |
Collaboration Agreements
The Company assess whether its collaboration agreements are subject to ASC Topic 808, Collaborative Arrangements (Topic 808) based on whether they involve joint operating activities and whether both parties have active participation in the arrangement and are exposed to significant risks and rewards. To the extent that the arrangement falls within the scope of Topic 808, the Company will apply by analogy the unit of account guidance under Topic 606 to identify distinct performance obligations, and then determine whether a customer relationship exists for each distinct performance obligation. If the Company determines a performance obligation within the arrangement is with a customer, the Company applies the guidance in Topic 606. If a portion of a distinct bundle of goods or services within an arrangement is not with a customer, then the unit of account is not within the scope of Topic 606, and the recognition and measurement of that unit of account shall be based on analogy to authoritative accounting literature or, if there is no appropriate analogy, a reasonable, rational, and consistently applied accounting policy election.
The terms of such arrangements typically include payments to the Company for one or more of the following: up-front fees; development and regulatory payments; product supply services; research and development cost reimbursements; profit-sharing arrangements; and royalties on certain products if they are successfully commercialized. As part of the accounting for these arrangements, the Company develops assumptions that require judgment to determine the standalone selling price for each performance obligation identified in the contract. These key assumptions may include forecasted revenues, clinical development timelines and costs, reimbursement rates for personnel costs, discount rates and probabilities of technical and regulatory success.
Up-front License Fees: If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company would recognize revenues from nonrefundable up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license, which generally would occur at or near the inception of the contract. For licenses that are bundled with other promises, the Company would utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenues from nonrefundable up-front fees. The Company will evaluate the measure of progress at the end of each reporting period and, if necessary, adjust the measure of performance and related revenue recognition.
Research and Development Milestone Payments: At the inception of each arrangement that includes development milestone payments, the Company will evaluate whether the milestones are considered probable of being reached and estimate the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the Company’s or the licensee’s control, such as regulatory approvals, are not considered probable of being achieved until uncertainty associated with the approvals has been resolved. The transaction price is then allocated to each performance obligation, on a relative standalone selling price basis, for which the Company will recognize revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, the Company re-evaluates the probability of achieving such development and regulatory milestones and any related variable consideration constraint, and if necessary, adjust the Company’s estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis.
Research and Development Cost Reimbursements: The Company’s collaboration arrangements may include promises of future clinical development and drug safety services, as well as participation on certain joint committees. When such services are provided to a customer or partner, and they are distinct from the licenses provided to the Company’s collaboration partners, these promises are accounted for as a separate performance obligation which the Company estimates using internal development costs incurred and projections through the term of the arrangements. The Company records revenues for these services as the performance obligations are satisfied over time based on measure of progress. However, if the Company concludes that its collaboration partner is not a customer for those collaborative research and development activities, it presents such payments as a reduction of research and development expenses.
| F-12 |
Research
and Development Arrangement: Under the terms of our research and development agreement with the CFF Agreement, the Company did not
account for this arrangement in accordance with Topic 606. However, the Company has determined that it is a partner under a collaboration
agreement as it shares in the risks and rewards that would be received if the product is successful and commercialized. Therefore the
funds received under the terms of this agreement will be recorded as reimbursements of research and development costs and reduce the
research and development expenses in the Company’s Statements of Operations and Comprehensive Loss. The Company records the reimbursements
for certain materials and other research and development costs associated with the agreement when it is probable that a significant reversal
in the amount of cumulative costs have been recognized. During the years ended December 31, 2023 and 2022, the Company recognized $
Research and development expenses
Research and development expenses primarily consist of costs associated with the preclinical and clinical development of our product candidate portfolio, including the following:
| ● | external research and development expenses incurred under arrangements with third parties, such as contract research organizations (“CROs”) and other vendors and contract manufacturing organizations (“CMOs”) for the production of drug substance and drug product; and |
| ● | employee-related expenses, including salaries, benefits and share-based compensation expense. |
Research and development expenses also include costs of acquired product licenses and related technology rights where there is no alternative future use, costs of prototypes used in research and development, consultant fees and amounts paid to certain of our collaborative partners.
All research and development expenses are charged to operations as incurred in accordance with FASB ASC Topic 730, Research and Development. The Company accounts for non-refundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been received, rather than when the payment is made.
Accrued Research and Development Expenses
As part of the process of preparing the Company’s financial statements, the Company is required to estimate its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of the actual cost. Certain of the Company’s service providers invoice the Company monthly in arrears for services performed or when contractual milestones are met. The Company makes estimates of its accrued expenses as of each balance sheet date in its financial statements based on facts and circumstances known to the Company at that time. The Company periodically confirms the accuracy of its estimates with the service providers and adjust if necessary. The significant estimates in the Company’s accrued research and development expenses are related to expenses incurred with respect to CROs, CMOs and other vendors in connection with research and development and manufacturing activities.
The Company bases its expense related to CROs and CMOs on its estimates of the services received and efforts expended pursuant to quotations and contracts with such vendors that conduct research and development and manufacturing activities on its behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to the Company’s vendors will exceed the level of services provided and result in a prepayment of the applicable research and development or manufacturing expense. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from its estimate, the Company adjust the accrual or prepaid expense accordingly. Although the Company does not expect its estimates to be materially different from amounts actually incurred, the Company’s understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in us reporting amounts that are too high or too low in any particular period. There have been no material changes in estimates for the periods presented.
| F-13 |
Patent expenses
Legal fees and other direct costs incurred in obtaining and protecting patents are also expensed as incurred and are included in general and administrative expenses in the consolidated statements of operations.
Other comprehensive loss
Other comprehensive loss consists of net gains/(losses) and unrealized losses on marketable debt securities available-for-sale and is presented in the Consolidated Statements of Operations and Comprehensive Loss.
Recent accounting pronouncements
In November 2023, the FASB issued Accounting Standard Update (“ASU”) No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (“ASU 2023-07”). This ASU requires disclosure of significant segment expenses that are regularly provided to the chief operating decision maker (“CODM”), an amount for other segment items with description of composition, and disclosure of the title and position of the CODM. ASU 2023-17 is effective for annual periods beginning after December 31, 2023 and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted and this ASU should be applied retrospectively to each period presented in the financial statements. Since the Company views its operations and manages its business in one operating and reporting segment, the Company believes this additional disclosure will not have an impact on its consolidated financial statements and related disclosures. As a result, we intend to adopt the provision of this ASU in the fourth quarter of 2024.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclousres (“ASU 2023-09”). This ASU requires public business entities on an annual basis to disclose specific categories in a tabular rate reconciliation and provide additional information for reconciling items that meet a five percent quantitative threshold. Additionally, the ASU requires all entities to disclose the amount of income taxed paid disaggregated by federal, state, and foreign taxes, as well as individual jurisdictions where income taxes paid are equal to or greater than five percent of total income taxes paid. ASU 2023-09 is effective for annual periods beginning after December 31, 2024. Early adoption is permitted and this ASU should be applied on a prospective basis, a retrospective application permitted in the financial statements. The Company is currently evaluating the impact of the new standard on our consolidated financial statements and related disclosures. As a result, we intend to adopt the provisions of this ASU in the fourth quarter of 2025.
Note 4 – Cash, Cash Equivalents, Restricted Cash and Marketable Debt Securities
The Company considers all highly liquid financial instruments with original maturities of three months or less when purchased to be cash and cash equivalents and all investments with maturities of greater than three months from date of purchase are classified as marketable debt securities. Cash and cash equivalents consisted of cash in bank checking and savings accounts, money market funds and short-term U.S. treasury bonds that mature within three months of settlement date.
Cash, Cash Equivalents and Restricted Cash
The
Company presents restricted cash with cash and cash equivalents in the Consolidated Statements of Cash Flows. Restricted cash at each
of December 31, 2023 and 2022 of $
| F-14 |
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported in the Consolidated Balance Sheets to the total of the amounts in the Consolidated Statements of Cash Flows as of December 31, 2023, December 31, 2022 and December 31, 2021:
December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Cash and cash equivalents | $ | $ | $ | |||||||||
| Restricted cash included in current/non-current assets | ||||||||||||
| Cash, cash equivalents and restricted cash in the statement of cash flows | $ | $ | $ | |||||||||
Marketable Debt Securities
The
Company has classified its investments in marketable debt securities as available-for-sale and as a current asset. The Company’s
investments in marketable debt securities are carried at fair value, with unrealized gains and losses included as a separate component
of stockholders’ equity. Unrealized losses and gains are classified as other comprehensive (loss)/income and costs are determined
on a specific identification basis. Realized gains and losses from marketable debt securities are recorded in other income, net. The
Company did not incur any realized gains and losses during the years ended December 31, 2023 and 2022. For the years ended December 31,
2023 and 2022, the Company recorded unrealized gains/(losses) of $
The following tables summarize the Company’s marketable debt securities as of December 31, 2023:
| Amortized | Unrealized | Unrealized | ||||||||||||||
| Cost | Gain | (Loss) | Fair Value | |||||||||||||
| U.S. Treasury Bonds | $ | $ | $ | ( | ) | $ | ||||||||||
| U.S. Government Notes | ( | ) | ||||||||||||||
| Total marketable debt securities | $ | $ | $ | ( | ) | $ | ||||||||||
All debt securities classified as available-for-sale are due to mature within one year of December 31, 2023.
| Amortized Cost | Unrealized Gain | Unrealized (Loss) | Fair Value | |||||||||||||
| U.S. Treasury Bonds | $ | $ | $ | ( | ) | $ | ||||||||||
| U.S. Government Notes | ( | ) | ||||||||||||||
| Corporate Debt Securities | ( | ) | ||||||||||||||
| Total marketable debt securities | $ | $ | $ | ( | ) | $ | ||||||||||
Maturities of debt securities classified as available-for-sale were as follows at December 31, 2022:
| Fair Value | ||||
| Due within one year | $ | |||
| Due after one year through five years | ||||
| $ | ||||
The Company determined that the unrealized (losses) and gains are temporary as of December 31, 2023 and 2022. Unrealized (losses) and gains generally are the result of increases in the risk premiums required by market participants rather than an adverse change in cash flows for a fundamental weakness in the credit quality of the issuer or underlying assets.
| F-15 |
Note 5 - Fair Value Measurements
The Company uses the fair value hierarchy to measure the value of its financial instruments. The fair value hierarchy is based on inputs to valuation techniques that are used to measure fair value that are either observable or unobservable. Observable inputs reflect assumptions market participants would use in pricing an asset or liability based on market data obtained from independent sources, while unobservable inputs reflect a reporting entity’s pricing based upon its own market assumptions. The basis for fair value measurements for each level within the hierarchy is described below:
| ● | Level 1 – Quoted prices for identical assets or liabilities in active markets. |
| ● | Level 2 – Quoted prices for identical or similar assets and liabilities in markets that are not active; or other model-derived valuations whose inputs are directly or indirectly observable or whose significant value drivers are observable. |
| ● | Level 3 – Valuations derived from valuation techniques in which one or more significant inputs to the valuation model are unobservable and for which assumptions are used based on management estimates. |
The Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible as well as considers counterparty credit risk in its assessment of fair value.
The carrying amounts of cash equivalents, current portion of restricted cash, prepaid expenses and other current assets, accounts payable, current portion of lease liabilities and accrued expenses approximate fair value due to the short-term nature of these instruments.
A summary of the assets and liabilities carried at fair value in accordance with the hierarchy defined above is as follows:
| Fair Value Hierarchy | ||||||||||||||||
| December 31, 2023 | Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Assets | ||||||||||||||||
| Marketable Debt Securities: | ||||||||||||||||
| U.S. Treasury Bonds | $ | $ | $ | $ | ||||||||||||
| U.S. Government Notes | ||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||
| Fair Value Hierarchy | ||||||||||||||||
| December 31, 2022 | Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Assets | ||||||||||||||||
| Marketable Debt Securities: | ||||||||||||||||
| U.S. Treasury Bonds | $ | $ | $ | $ | ||||||||||||
| U.S. Government Notes | ||||||||||||||||
| Corporate Debt Securities | ||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||
U.S. treasury bonds are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices for identical assets in active markets. Marketable debt securities consisting of U.S. government notes and corporate debt securities are classified as Level 2 and are valued using quoted market prices in markets that are not active.
| F-16 |
Note 6 – Leasehold Improvements and Equipment
Leasehold improvements and equipment, summarized by major category, consist of the following for the years ended December 31, 2023 and 2022:
December 31, 2023 | December 31, 2022 | |||||||
| Equipment | $ | $ | ||||||
| Leasehold improvements | ||||||||
| Total | ||||||||
| Less: accumulated depreciation and amortization | ||||||||
| Leasehold improvements and equipment, net | $ | $ | ||||||
Depreciation
and amortization expense for the years ended December 31, 2023 and 2022 was $
Note 7 – Accrued Expenses and Other Liabilities
Accrued expenses and other liabilities, summarized by major category, consist of the following for years ended December 31, 2023 and 2022:
| As of December 31, | ||||||||
| 2023 | 2022 | |||||||
| Payroll and incentives | $ | $ | ||||||
| General and administrative expenses | ||||||||
| Research and development expenses | ||||||||
| Deferred revenue * | ||||||||
| Other deferred liabilities ** | ||||||||
| Total | $ | $ | ||||||
| * | |
| ** |
Note 8 – Leases
The
Company has various lease agreements including leases of office space, a laboratory and manufacturing facility, and various equipment.
Operating and finance leases are presented in the Company’s consolidated balance sheets as right-of-use assets from leases, current lease liabilities and long-term lease liabilities. The assets and liabilities from our leases are recognized at the lease commencement date based on the present value of remaining lease payments over the lease term using the Company’s incremental borrowing rates or implicit rates, when readily determinable. Short-term leases, which have an initial term of 12 months or less, are not recorded on the balance sheet. As the Company’s operating leases do not provide implicit rates, the Company has utilized its incremental borrowing rate, determined based on the long-term borrowing costs of companies with similar credit profiles, to record its lease obligations. The Company’s finance leases provide readily determinable implicit rates. For operating leases, the Company recognizes the minimum rental expense on a straight-line basis based on the fixed components of a lease arrangement. The Company will amortize this expense over the term of the lease beginning with the lease commencement date.
| F-17 |
Operating lease obligations
On
November 1, 2013, the Company entered into a
On
December 15, 2016, the Company entered into a lease of laboratory and manufacturing space in Bridgewater, New Jersey.
The lease began August 2017. The monthly rent started at approximately $
The
Company incurred lease expense for its operating leases of $
Finance Leases
The
Company incurred interest expense on its finance leases of $
The following table presents information about the amount and timing of liabilities arising from the Company’s operating leases and finance leases as of December 31, 2023:
| Maturity of Lease Liabilities | Operating Lease Liabilities | Finance Lease Liabilities | ||||||
| 2024 | $ | $ | ||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| Thereafter | ||||||||
| Total undiscounted operating lease payments | $ | $ | ||||||
| Less: Imputed interest | ||||||||
| Present value of operating lease liabilities | $ | $ | ||||||
| Weighted average remaining lease term in years | ||||||||
| Weighted average discount rate | % | % | ||||||
The following table presents information about the amount and timing of liabilities arising from the Company’s operating leases and finance leases as of December 31, 2022:
| Maturity of Lease Liabilities | Operating Lease Liabilities | Finance Lease Liabilities | ||||||
| 2023 | $ | $ | ||||||
| 2024 | ||||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| Thereafter | ||||||||
| Total undiscounted operating lease payments | $ | $ | ||||||
| Less: Imputed interest | ||||||||
| Present value of operating lease liabilities | $ | $ | ||||||
| Weighted average remaining lease term in years | ||||||||
| Weighted average discount rate | % | % | ||||||
| F-18 |
Note 9 – Revenue Recognition, Collaboration Agreements, and Other Research and Development Agreements
BioNTech Research Collaboration
On
April 8, 2022, the Company entered into the BioNTech Agreement to evaluate the combination of mRNA formats utilizing the Company’s
proprietary LNC Platform. Under the terms of the BioNTech Agreement, the Company received an exclusivity fee in the amount of $
The Company assessed the BioNTech Agreement under ASC 808 Collaboration Arrangements and ASC 606 Revenue from Contracts with Customers (“ASC 606”) and concluded that the contract counterparty, BioNTech SE, is a customer based on the arrangement structure. The Company identified two material promises to deliver under the contract: (1) grant of an exclusive research license and (2) clinical research services. However, given the nature of the promises, the license and research services are not considered to be distinct from each other within the context of the contract. The Company therefore concluded that there is one combined performance obligation for both the license and research services.
The
$
For
the year ended December 31, 2023, $
Cystic Fibrosis Foundation Therapeutics Development Award
On
November 19, 2020, the Company entered into an award agreement (the “CFF Agreement”) with the Cystic Fibrosis Foundation
(“CFF”), pursuant to which it received a Therapeutics Development Award of up to $
As
of December 31, 2023, the Company has received $
| F-19 |
Genentech Feasibility Study Agreement
On
December 12, 2019, the Company entered into the Genentech Agreement which involves the development of oral formulations using the
Company’s LNC Platform. Under the terms of the Genentech Agreement, Genentech paid the Company a total of $
License Agreement
Through
the acquisition of Aquarius, the Company acquired a license from Rutgers University, The State University of New Jersey (successor in
interest to the University of Medicine and Dentistry of New Jersey) for certain patents related to the LNC Platform (the “License
Agreement”).
Note 10 – Commitments
Royalty payment rights
Pursuant
to the terms of the Certificate of Designations of Preferences, Rights and Limitations (the “Certificate of Designations”)
for our Series A Preferred Stock, the Company may be required to pay royalties of up to $
Employment agreements
The Company also has employment agreements with certain employees which require the funding of a specific level of payments, if certain events, such as a change in control, termination without cause or retirement, occur.
Other normal business operating agreements
In addition, in the course of normal business operations, the Company enters into agreements with contract service providers to assist in the performance of research and development and manufacturing activities. Expenditures to these third parties represent significant costs in clinical development and may require upfront payments and long-term commitments of cash. Subject to required notice periods and obligations under binding purchase orders, the Company can elect to discontinue the work under these agreements at any time.
Legal proceedings
The Company is not currently a party to any legal proceedings, and the Company is not aware of any claims or actions pending or threatened against its business. In the future, the Company might from time to time become involved in litigation relating to claims arising from our ordinary course of business.
| F-20 |
Note 11 – Income Taxes
The Company utilizes the liability method of accounting for deferred income taxes. Under this method, deferred tax liabilities and assets are recognized for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities. A valuation allowance is established against deferred tax assets when, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. The Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. As of December 31, 2023 and 2022, the Company does not believe any material uncertain tax positions were present. Accordingly, interest and penalties have not been accrued due to an uncertain tax position.
The components of the income tax provision are as follows:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Current expense (benefit): | ||||||||
| Federal | $ | $ | ||||||
| State | ||||||||
| Foreign | ||||||||
| Total current expense (benefit): | $ | $ | ||||||
| Deferred expense (benefit): | ||||||||
| Federal | $ | $ | ||||||
| State | ||||||||
| Foreign | ||||||||
| Total deferred expense (benefit): | $ | $ | ||||||
| Total income tax expense (benefit): | $ | $ | ||||||
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A reconciliation of the statutory U.S. federal rate to the Company’s effective tax rate is as follows:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Income at U.S. Statutory Rate | % | % | ||||||
| State Taxes, net of Federal benefit | % | % | ||||||
| Permanent Differences | ( | )% | ( | )% | ||||
| Tax Credits | % | % | ||||||
| Valuation Allowance | ( | )% | ( | )% | ||||
| % | % | |||||||
The Company has no current income taxes payable other than certain state minimum taxes which are included in general and administrative expenses.
Significant components of the Company’s deferred tax assets (liabilities) for 2023 and 2022 consist of the following:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Share-based Compensation | $ | $ | ||||||
| Depreciation and Amortization | ( | ) | ( | ) | ||||
| Accrued Liability | ||||||||
| Net Operating Loss Carry-forwards | ||||||||
| R&D Credit Carryforwards | ||||||||
| R&D Section 174 Costs | ||||||||
| Other | ( | ) | ||||||
| IPR&D | ( | ) | ( | ) | ||||
| ROU Asset | ( | ) | ( | ) | ||||
| ROU Liability | ||||||||
| Total Deferred tax assets | $ | $ | ||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax asset (liability) | $ | ( | ) | $ | ( | ) | ||
| F-21 |
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security (CARES) Act was signed into law making several changes to the Internal Revenue Code. The changes include but are not limited to: increasing the limitation on the amount of deductible interest expense, allowing companies to carryback certain net operating losses, and increasing the amount of net operating loss carryforwards that corporations can use to offset taxable income. The tax law changes in the Act did not have a material impact on the Company’s income tax provision.
In
assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all
of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of
taxable income during the periods in which the temporary differences representing net future deductible amounts become deductible, and
is impacted by the Company’s ability to carryforward losses to years in which the Company has taxable income. Due to the Company’s
history of losses and lack of other positive evidence to support taxable income, the Company has recorded a valuation allowance against
those deferred tax assets that are not expected to be realized. The valuation allowances were $
As
of December 31, 2023, the Company had Federal net operating loss carryforwards of $
Utilization of the net operating losses and general business tax credits carryforwards may be subject to a substantial limitation under Sections 382 and 383 of the Internal Revenue Code of 1986 due to changes in ownership of the Company that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating losses and general business tax credits carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. The Company has not completed a study to determine whether it had undergone an ownership change since the Company’s inception.
Under the Tax Cuts and Jobs Act of 2017, research and development costs are no longer fully deductible and are required to be capitalized and amortized for U.S. tax purposes effective January 1, 2022. The mandatory capitalization requirement increases our deferred tax assets.
Sale of net operating losses (NOLs) & tax credits
The
Company recognized $
Note 12 – Stockholders’ Equity
At-The-Market Equity Offering
On
July 2, 2020, the Company entered into an At-The-Market Sales Agreement (the “Sales Agreement”) with BTIG, LLC (“BTIG”),
pursuant to which the Company may offer and sell, from time to time, through BTIG, as sales agent and/or principal, shares of its common
stock having an aggregate offering price of up to $
| F-22 |
Common Stock
On
February 8, 2022, the Company issued unregistered shares of its common stock to Rutgers, The State University of New Jersey (“Rutgers”),
as partial consideration pursuant to the Second Amended and Restated Exclusive License Agreement between
Preferred Stock
In accordance with the Certificate of Incorporation, the Company is authorized to issue preferred shares at a par value of $.
Warrants
As of December 31, 2023, the Company did not have any outstanding warrants to purchase shares of common stock. A summary of warrants outstanding as of December 31, 2023 and 2022 is presented below:
| Shares | ||||
| Outstanding at December 31, 2021 | ||||
| Issued | ||||
| Exercised | ( | ) | ||
| Expired | ( | ) | ||
| Outstanding at December 31, 2022 | ||||
| Issued | ||||
| Exercised | ||||
| Expired | ( | ) | ||
| Outstanding at December 31, 2023 | ||||
Note 13 – Accumulated Other Comprehensive Income/(Loss)
The following table summarizes the changes in accumulated other comprehensive income/(loss) by components during the years ended December 31, 2023 and 2022:
| Net Unrealized Gains/(Losses) on Available-for-Sale Securities | Accumulated Other Comprehensive Income/(Loss) | |||||||
| Balance, December 31, 2021 | $ | ( | ) | $ | ( | ) | ||
| Net unrealized loss on securities available-for-sale | ( | ) | ( | ) | ||||
| Balance, December 31, 2022 | $ | ( | ) | $ | ( | ) | ||
| Net unrealized gain on securities available-for-sale | ||||||||
| Balance, December 31, 2023 | $ | ( | ) | $ | ( | ) | ||
All components of accumulated other comprehensive income/(loss) are net of tax.
| F-23 |
The Company’s Amended and Restated 2013 Equity Compensation Plan (the “Plan”) provides for the granting of incentive stock options, nonqualified stock options, restricted stock units, performance units, and stock purchase rights to eligible employees, officers, non-employee directors and other individual service providers. Options under the Plan may be of the shares on the date of grant as determined by the Compensation Committee of the Board of Directors. The Compensation Committee determines the period over which the options become exercisable subject to certain restrictions as defined in the Plan, with the current outstanding options generally vesting over or years. . As of December 31, 2023, the Company had shares of common stock authorized for issuance under the Plan.
With the approval of the Board of Directors and a majority of shareholders, effective May 8, 2014, the Plan was amended and restated. The amendment provides for an automatic increase in the number of shares of common stock available for issuance under the Plan each January (with Board approval), commencing in an amount up to four percent (%) of the total number of shares of common stock outstanding on the preceding December 31st.
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Research and Development | $ | $ | ||||||
| General and Administrative | ||||||||
| Total | $ | $ | ||||||
| Awards Reserved for Issuance | Awards Issued & Exercised | Awards Available for Grant | ||||||||||
| 2013 Equity Compensation Plan (in thousands) | * | ** | ||||||||||
| * | |
| ** |
Stock Options
| Number of Options | Weighted Average Exercise Price | Weighted Average Contractual Term in Years | ||||||||||
| Outstanding at January 1, 2022 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ( | ) | ||||||||||
| Forfeited | ( | ) | ||||||||||
| Expired | ( | ) | ||||||||||
| Outstanding at December 31, 2022 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ||||||||||||
| Forfeited | ( | ) | ||||||||||
| Expired | ( | ) | ||||||||||
| Outstanding at December 31, 2023 | $ | |||||||||||
| F-24 |
| Range of Exercise Prices | Number Outstanding | Weighted Average Exercise Price Per Share | ||||||
| $ - $ | $ | |||||||
| $ - $ | $ | |||||||
| $ - $ | $ | |||||||
| $ - $ | $ | |||||||
| $ | ||||||||
As of December 31, 2023, the number of vested shares underlying outstanding options was at a weighted average exercise price of $. The aggregate intrinsic value of in-the-money options outstanding as of December 31, 2023 was $. The aggregate intrinsic value is calculated as the difference between the Company’s closing stock price of $ on December 31, 2023, and the exercise price of options, multiplied by the number of options. As of December 31, 2023, there was $ of total unrecognized share-based compensation. Such costs are expected to be recognized over a weighted average period of approximately years.
from date of grant. Options granted to employees prior to 2018 vest in equal monthly installments over three years. Beginning in 2018, options granted to employees vest over four years, with % of the shares vesting on the first annual anniversary of grant and the remaining shares vesting in 36 equal monthly installments over the following 3 years.
The resulting compensation expense for stock options is generally recognized on a straight-line basis over the requisite service period of the award. The following weighted-average assumptions were used to calculate share-based compensation for the comparative periods presented:
| For the Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Volatility | % - | % | % - | % | ||||
| Risk-free interest rate | % - | % | % - | % | ||||
| Dividend yield | % | % | ||||||
| Expected life | years | years | ||||||
The Company does not have sufficient historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior. Hence, the Company uses the “simplified method” described in Staff Accounting Bulletin (SAB) 107 to estimated the expected term of share option grants.
The expected stock price volatility assumption is based on the Company’s historical stock price volatility.
Note 15 – Subsequent Events
None.
| F-25 |