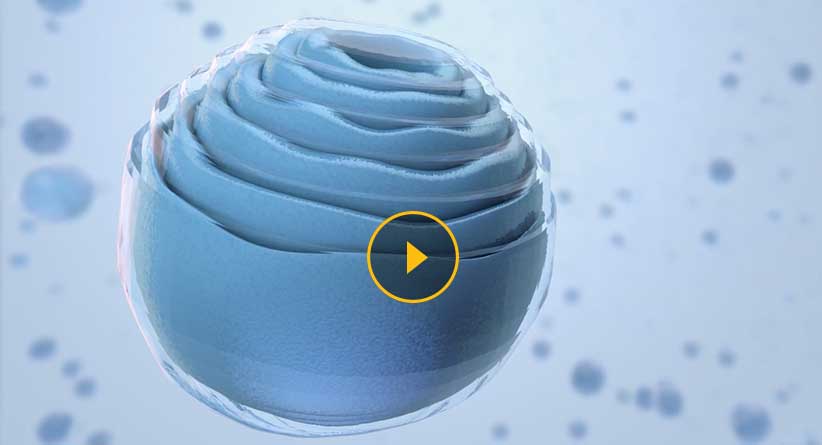
Drug Encapsulation
- The cochleate is prepared using naturally occurring phospholipids and calcium.
- When calcium interacts with the negatively charged phospholipids, they spiral into non-toxic, highly stable crystalline units with multiple layers and little to no internal aqueous space.
- The active drug molecules become trapped within the layers, where they are protected from water and harmful external elements.

Intracellular Release
- The cochleate is delivered into the body and directly targets the clinically relevant cells.
- It then fuses with the cell membrane in a non-destructive manner.
- The low calcium environment of the cell’s interior causes the cochleate to unlock and unwind, releasing the drug into the target cell.
Key Features
Our LNC technology was engineered with core characteristics that transform the delivery of certain therapeutics.
SAFE
Delivers molecules in a natural, non-toxic and non-destructive manner without provoking an immune response.
TARGETED
Physiologically targets “stressed” cells with high levels of externalized phosphatidylserine and enters them directly, helping to maximize efficacy.
ORALLY BIOAVAILABLE
Enables oral administration and easy absorption into the circulatory system through the lymphatics.
STABLE
Resists water penetration, environmental attacks and degradation in the GI tract; increases the stability of therapeutics including small oligonucleotides.
Traditional vs. LNC Vehicles
We are addressing the delivery challenges of current therapies.
We intend to accomplish three primary goals through our LNC technology – the first being the improvement of the safety profile of drugs that cause excessive off-target organ toxicity. Second, we are facilitating targeted intracellular delivery – an essential method for small oligonucleotide therapies. Finally, we are delivering small oligonucleotides and insoluble small molecules via oral delivery.

Traditional Model
- Requires high plasma levels
- Low amounts of the drug molecules can penetrate the protective cell layers, while the rest are left circulating in the system
- Potentially results in off-target organ toxicity

LNC Model
- Lower plasma levels of active drug
- Enters cells directly, releasing the drug content into the infected cell membrane
- Likely to result in less systemic toxicity